Recifin A, Initial Example of the Tyr-Lock Peptide Structural Family, Is a Selective Allosteric Inhibitor of Tyrosyl-DNA Phosphodiesterase I.
Krumpe, L.R.H., Wilson, B.A.P., Marchand, C., Sunassee, S.N., Bermingham, A., Wang, W., Price, E., Guszczynski, T., Kelley, J.A., Gustafson, K.R., Pommier, Y., Rosengren, K.J., Schroeder, C.I., O'Keefe, B.R.(2020) J Am Chem Soc 142: 21178-21188
- PubMed: 33263997
- DOI: https://doi.org/10.1021/jacs.0c10418
- Primary Citation of Related Structures:
6XN9 - PubMed Abstract:
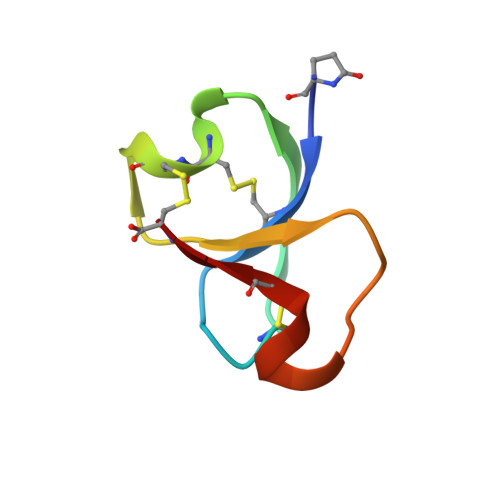
Tyrosyl-DNA phosphodiesterase 1 (TDP1) is a molecular target for the sensitization of cancer cells to the FDA-approved topoisomerase inhibitors topotecan and irinotecan. High-throughput screening of natural product extract and fraction libraries for inhibitors of TDP1 activity resulted in the discovery of a new class of knotted cyclic peptides from the marine sponge Axinella sp. Bioassay-guided fractionation of the source extract resulted in the isolation of the active component which was determined to be an unprecedented 42-residue cysteine-rich peptide named recifin A. The native NMR structure revealed a novel fold comprising a four strand antiparallel β-sheet and two helical turns stabilized by a complex disulfide bond network that creates an embedded ring around one of the strands. The resulting structure, which we have termed the Tyr-lock peptide family, is stabilized by a tyrosine residue locked into three-dimensional space. Recifin A inhibited the cleavage of phosphodiester bonds by TDP1 in a FRET assay with an IC 50 of 190 nM. Enzyme kinetics studies revealed that recifin A can specifically modulate the enzymatic activity of full-length TDP1 while not affecting the activity of a truncated catalytic domain of TDP1 lacking the N-terminal regulatory domain (Δ1-147), suggesting an allosteric binding site for recifin A on the regulatory domain of TDP1. Recifin A represents both the first of a unique structural class of knotted disulfide-rich peptides and defines a previously unseen mechanism of TDP1 inhibition that could be productively exploited for potential anticancer applications.
Organizational Affiliation:
Basic Science Program, Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, Frederick, Maryland 21702, United States.