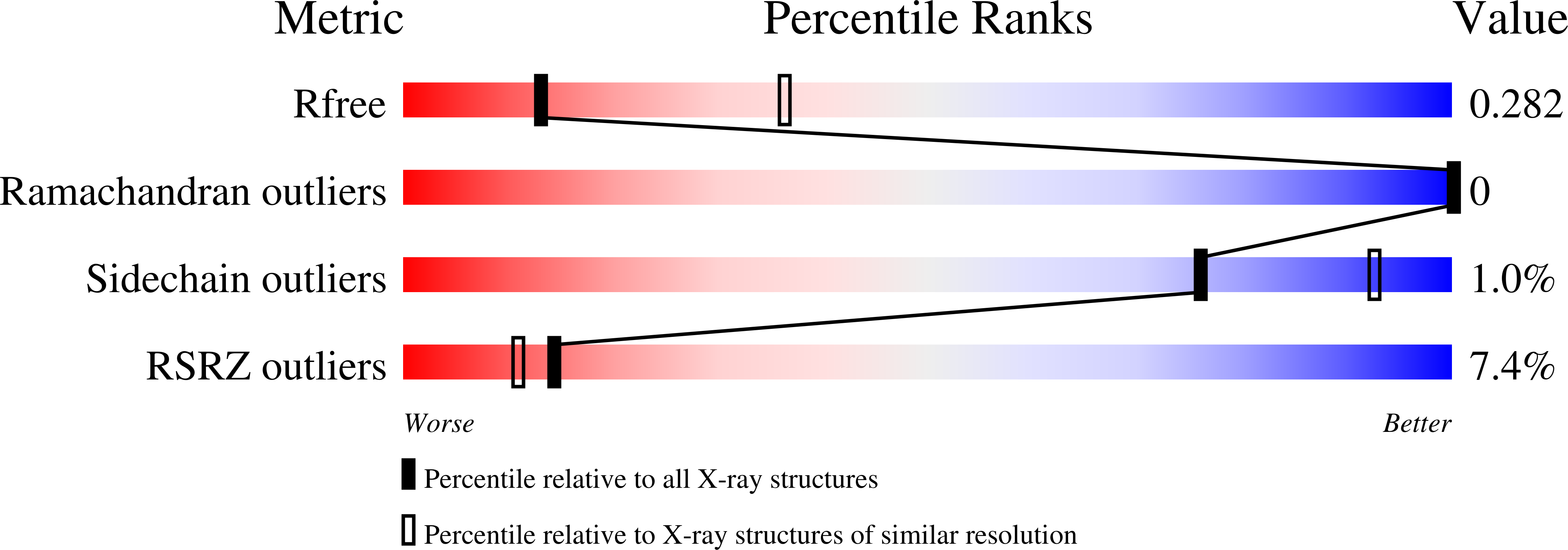Uncovering the basis of protein-protein interaction specificity with a combinatorially complete library.
Lite, T.V., Grant, R.A., Nocedal, I., Littlehale, M.L., Guo, M.S., Laub, M.T.(2020) Elife 9
- PubMed: 33107822
- DOI: https://doi.org/10.7554/eLife.60924
- Primary Citation of Related Structures:
6X0A - PubMed Abstract:
Protein-protein interaction specificity is often encoded at the primary sequence level. However, the contributions of individual residues to specificity are usually poorly understood and often obscured by mutational robustness, sequence degeneracy, and epistasis. Using bacterial toxin-antitoxin systems as a model, we screened a combinatorially complete library of antitoxin variants at three key positions against two toxins. This library enabled us to measure the effect of individual substitutions on specificity in hundreds of genetic backgrounds. These distributions allow inferences about the general nature of interface residues in promoting specificity. We find that positive and negative contributions to specificity are neither inherently coupled nor mutually exclusive. Further, a wild-type antitoxin appears optimized for specificity as no substitutions improve discrimination between cognate and non-cognate partners. By comparing crystal structures of paralogous complexes, we provide a rationale for our observations. Collectively, this work provides a generalizable approach to understanding the logic of molecular recognition.
Organizational Affiliation:
Department of Biology Massachusetts Institute of Technology, Cambridge, United States.