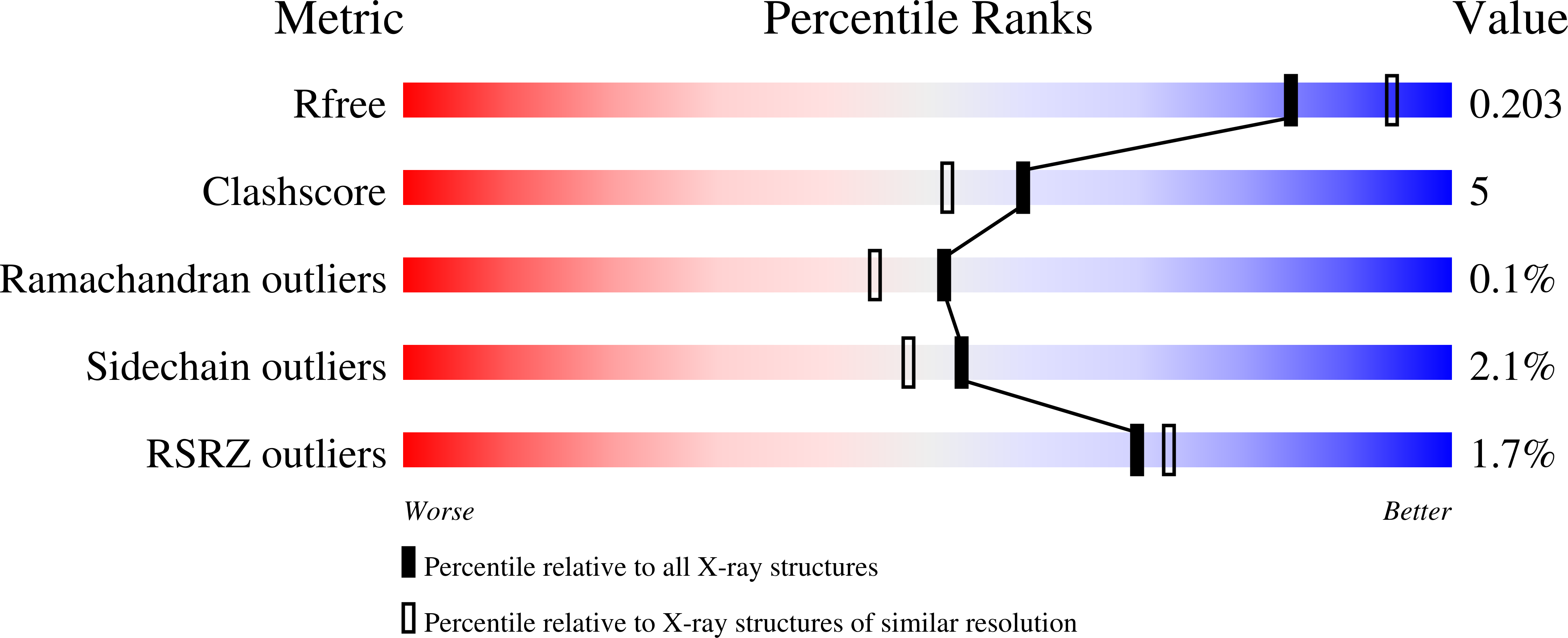
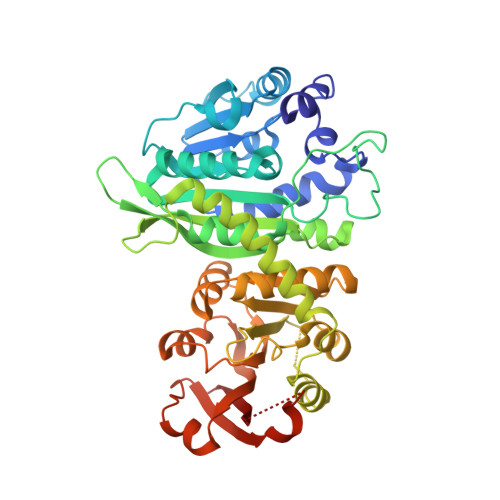
Structural and biochemical analysis of phosphoethanolamine methyltransferase from the pine wilt nematode Bursaphelenchus xylophilus.
Lee, S.G., Chung, M.S., DeMarsilis, A.J., Holland, C.K., Jaswaney, R.V., Jiang, C., Kroboth, J.H.P., Kulshrestha, K., Marcelo, R.Z.W., Meyyappa, V.M., Nelson, G.B., Patel, J.K., Petronio, A.J., Powers, S.K., Qin, P.R., Ramachandran, M., Rayapati, D., Rincon, J.A., Rocha, A., Ferreira, J.G.R.N., Steinbrecher, M.K., Yao, K., Zhang, E.J., Zou, A.J., Gang, M., Sparks, M., Cascella, B., Cruz, W., Jez, J.M.(2020) Mol Biochem Parasitol 238: 111291-111291
- PubMed: 32479776
- DOI: https://doi.org/10.1016/j.molbiopara.2020.111291
- Primary Citation of Related Structures:
6WLF - PubMed Abstract:
In free-living and parasitic nematodes, the methylation of phosphoethanolamine to phosphocholine provides a key metabolite to sustain phospholipid biosynthesis for growth and development. Because the phosphoethanolamine methyltransferases (PMT) of nematodes are essential for normal growth and development, these enzymes are potential targets of inhibitor design. The pine wilt nematode (Bursaphelenchus xylophilus) causes extensive damage to trees used for lumber and paper in Asia. As a first step toward testing BxPMT1 as a potential nematicide target, we determined the 2.05 Å resolution x-ray crystal structure of the enzyme as a dead-end complex with phosphoethanolamine and S-adenosylhomocysteine. The three-dimensional structure of BxPMT1 served as a template for site-directed mutagenesis to probe the contribution of active site residues to catalysis and phosphoethanolamine binding using steady-state kinetic analysis. Biochemical analysis of the mutants identifies key residues on the β1d-α6 loop (W123F, M126I, and Y127F) and β1e-α7 loop (S155A, S160A, H170A, T178V, and Y180F) that form the phosphobase binding site and suggest that Tyr127 facilitates the methylation reaction in BxPMT1.
Organizational Affiliation:
Department of Biology, Washington University in St. Louis, One Brookings Drive, St. Louis, MO, 63130, United States.