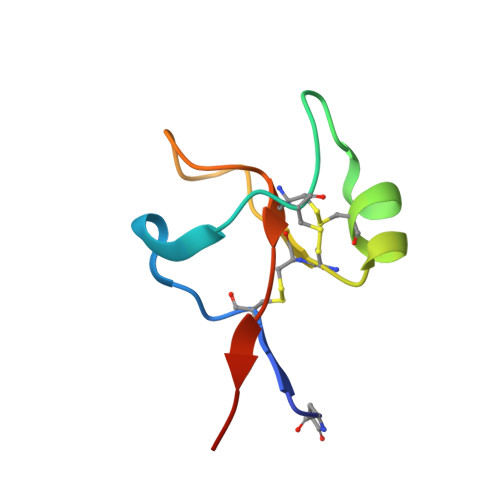
Trefoil factors share a lectin activity that defines their role in mucus.
Jarva, M.A., Lingford, J.P., John, A., Soler, N.M., Scott, N.E., Goddard-Borger, E.D.(2020) Nat Commun 11: 2265-2265
- PubMed: 32404934
- DOI: https://doi.org/10.1038/s41467-020-16223-7
- Primary Citation of Related Structures:
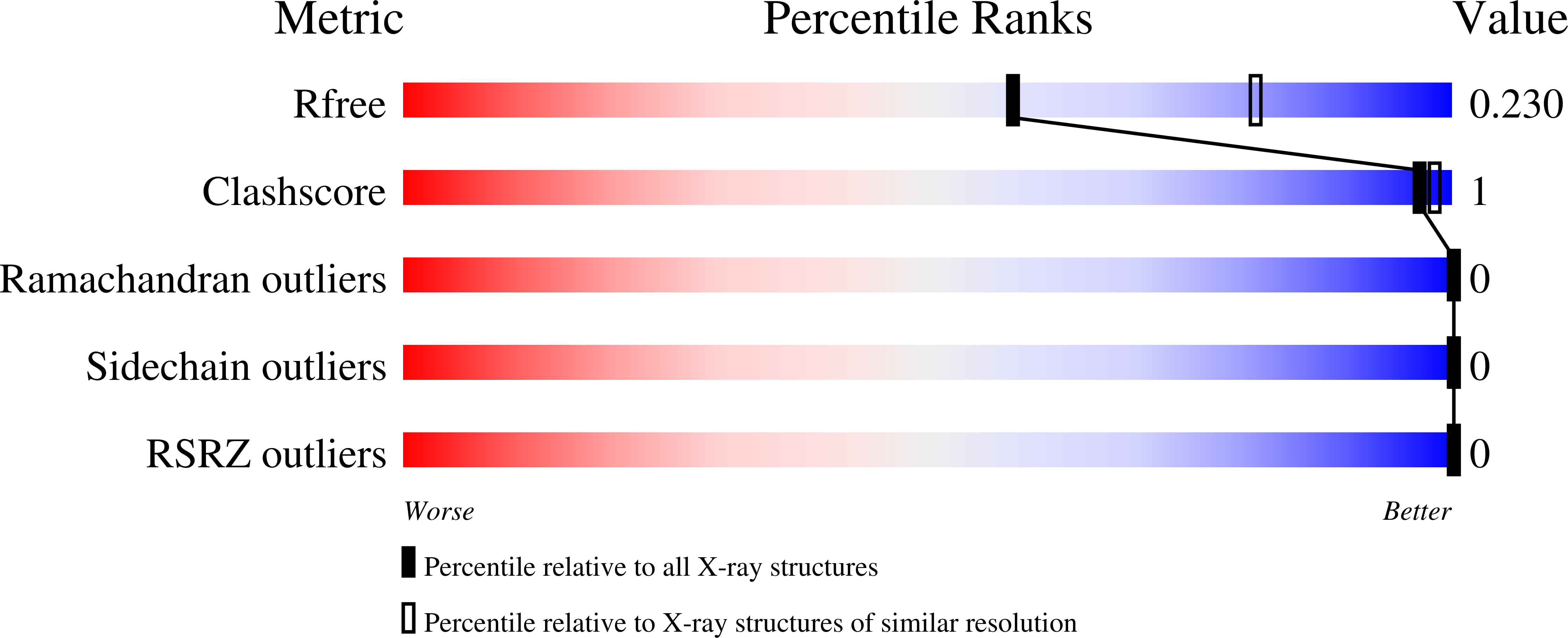
6V1C, 6V1D - PubMed Abstract:
The mucosal epithelium secretes a host of protective disulfide-rich peptides, including the trefoil factors (TFFs). The TFFs increase the viscoelasticity of the mucosa and promote cell migration, though the molecular mechanisms underlying these functions have remained poorly defined. Here, we demonstrate that all TFFs are divalent lectins that recognise the GlcNAc-α-1,4-Gal disaccharide, which terminates some mucin-like O-glycans. Degradation of this disaccharide by a glycoside hydrolase abrogates TFF binding to mucins. Structural, mutagenic and biophysical data provide insights into how the TFFs recognise this disaccharide and rationalise their ability to modulate the physical properties of mucus across different pH ranges. These data reveal that TFF activity is dependent on the glycosylation state of mucosal glycoproteins and alludes to a lectin function for trefoil domains in other human proteins.
Organizational Affiliation:
The Walter and Eliza Hall Institute of Medical Research, Parkville, VIC, 3052, Australia.