Antifungal symbiotic peptide NCR044 exhibits unique structure and multifaceted mechanisms of action that confer plant protection.
Velivelli, S.L.S., Czymmek, K.J., Li, H., Shaw, J.B., Buchko, G.W., Shah, D.M.(2020) Proc Natl Acad Sci U S A 117: 16043-16054
- PubMed: 32571919
- DOI: https://doi.org/10.1073/pnas.2003526117
- Primary Citation of Related Structures:
6U6G - PubMed Abstract:
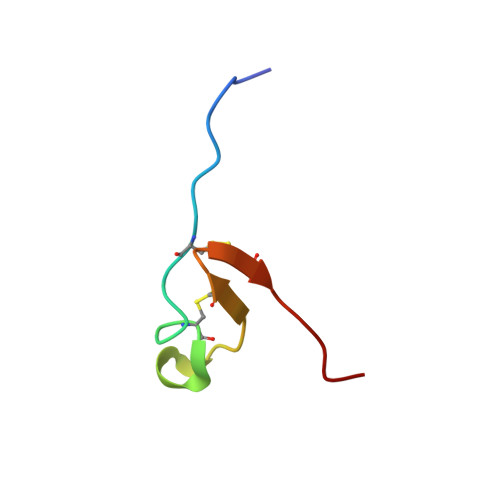
In the indeterminate nodules of a model legume Medicago truncatula , ∼700 nodule-specific cysteine-rich (NCR) peptides with conserved cysteine signature are expressed. NCR peptides are highly diverse in sequence, and some of these cationic peptides exhibit antimicrobial activity in vitro and in vivo. However, there is a lack of knowledge regarding their structural architecture, antifungal activity, and modes of action against plant fungal pathogens. Here, the three-dimensional NMR structure of the 36-amino acid NCR044 peptide was solved. This unique structure was largely disordered and highly dynamic with one four-residue α-helix and one three-residue antiparallel β-sheet stabilized by two disulfide bonds. NCR044 peptide also exhibited potent fungicidal activity against multiple plant fungal pathogens, including Botrytis cinerea and three Fusarium spp. It inhibited germination in quiescent spores of B. cinerea In germlings, it breached the fungal plasma membrane and induced reactive oxygen species. It bound to multiple bioactive phosphoinositides in vitro. Time-lapse confocal and superresolution microscopy revealed strong fungal cell wall binding, penetration of the cell membrane at discrete foci, followed by gradual loss of turgor, subsequent accumulation in the cytoplasm, and elevated levels in nucleoli of germlings. Spray-applied NCR044 significantly reduced gray mold disease symptoms caused by the fungal pathogen B. cinerea in tomato and tobacco plants, and postharvest products. Our work illustrates the antifungal activity of a structurally unique NCR peptide against plant fungal pathogens and paves the way for future development of this class of peptides as a spray-on fungistat/fungicide.
Organizational Affiliation:
Donald Danforth Plant Science Center, St Louis, MO 63132.