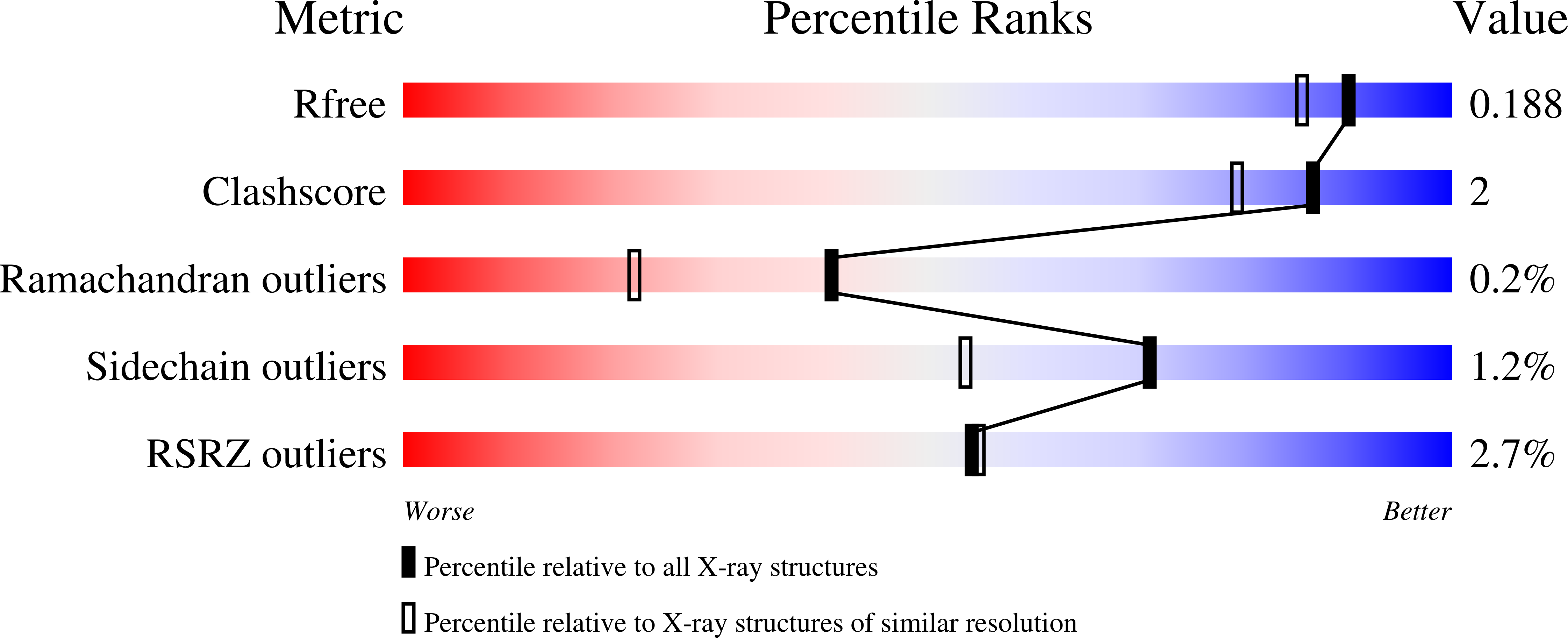
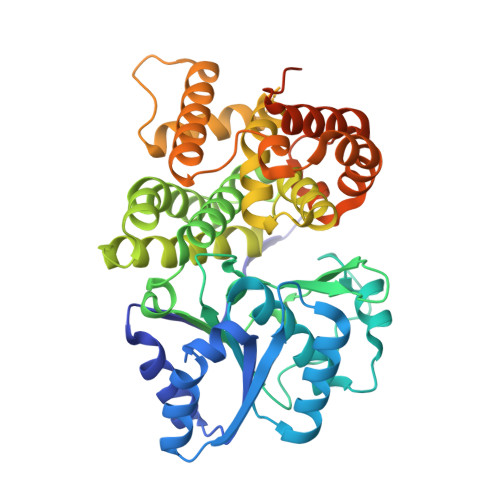
High-resolution structure of the alcohol dehydrogenase domain of the bifunctional bacterial enzyme AdhE.
Azmi, L., Bragginton, E.C., Cadby, I.T., Byron, O., Roe, A.J., Lovering, A.L., Gabrielsen, M.(2020) Acta Crystallogr F Struct Biol Commun 76: 414-421
- PubMed: 32880589
- DOI: https://doi.org/10.1107/S2053230X20010237
- Primary Citation of Related Structures:
6SCG, 6SCI - PubMed Abstract:
The bifunctional alcohol/aldehyde dehydrogenase (AdhE) comprises both an N-terminal aldehyde dehydrogenase (AldDH) and a C-terminal alcohol dehydrogenase (ADH). In vivo, full-length AdhE oligomerizes into long oligomers known as spirosomes. However, structural analysis of AdhE is challenging owing to the heterogeneity of the spirosomes. Therefore, the domains of AdhE are best characterized separately. Here, the structure of ADH from the pathogenic Escherichia coli O157:H7 was determined to 1.65 Å resolution. The dimeric crystal structure was confirmed in solution by small-angle X-ray scattering.
Organizational Affiliation:
Institute of Infection, Immunity and Inflammation, University of Glasgow, University Avenue, Glasgow G12 8QQ, United Kingdom.