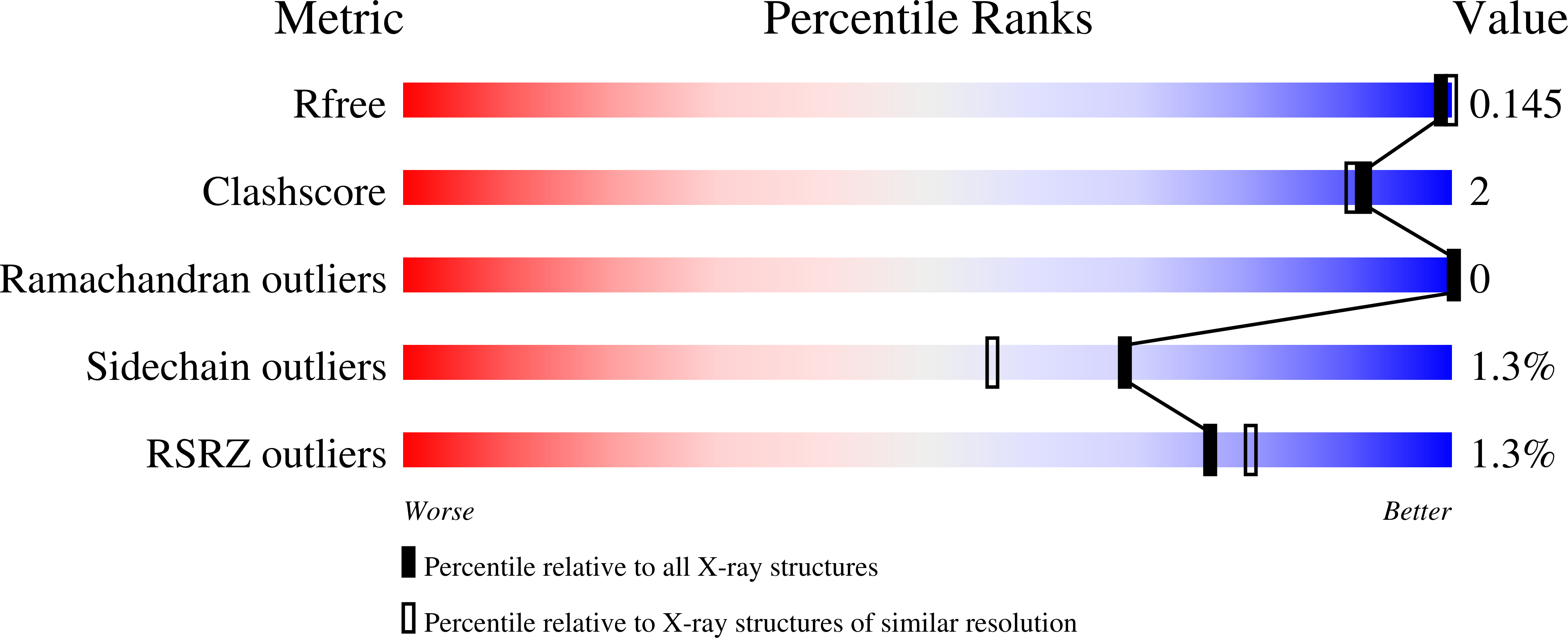
Crystal structure ofcis-aconitate decarboxylase reveals the impact of naturally occurring human mutations on itaconate synthesis.
Chen, F., Lukat, P., Iqbal, A.A., Saile, K., Kaever, V., van den Heuvel, J., Blankenfeldt, W., Bussow, K., Pessler, F.(2019) Proc Natl Acad Sci U S A 116: 20644-20654
- PubMed: 31548418
- DOI: https://doi.org/10.1073/pnas.1908770116
- Primary Citation of Related Structures:
6R6T, 6R6U - PubMed Abstract:
cis -Aconitate decarboxylase (CAD, also known as ACOD1 or Irg1) converts cis -aconitate to itaconate and plays central roles in linking innate immunity with metabolism and in the biotechnological production of itaconic acid by Aspergillus terreus We have elucidated the crystal structures of human and murine CADs and compared their enzymological properties to CAD from A. terreus Recombinant CAD is fully active in vitro without a cofactor. Murine CAD has the highest catalytic activity, whereas Aspergillus CAD is best adapted to a more acidic pH. CAD is not homologous to any known decarboxylase and appears to have evolved from prokaryotic enzymes that bind negatively charged substrates. CADs are homodimers, the active center is located in the interface between 2 distinct subdomains, and structural modeling revealed conservation in zebrafish and Aspergillus We identified 8 active-site residues critical for CAD function and rare naturally occurring human mutations in the active site that abolished CAD activity, as well as a variant (Asn152Ser) that increased CAD activity and is common (allele frequency 20%) in African ethnicity. These results open the way for 1) assessing the potential impact of human CAD variants on disease risk at the population level, 2) developing therapeutic interventions to modify CAD activity, and 3) improving CAD efficiency for biotechnological production of itaconic acid.
Organizational Affiliation:
Department Structure and Function of Proteins, Helmholtz Centre for Infection Research, 38124 Braunschweig, Germany.