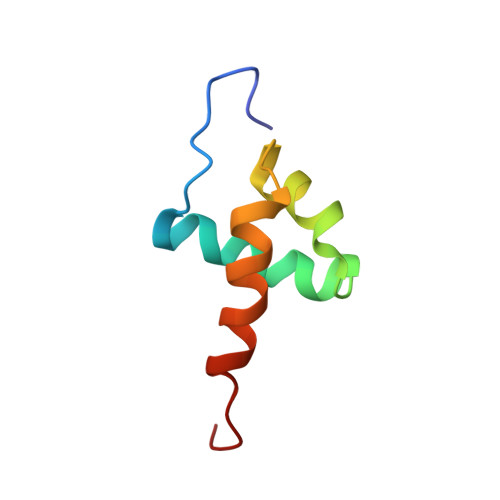
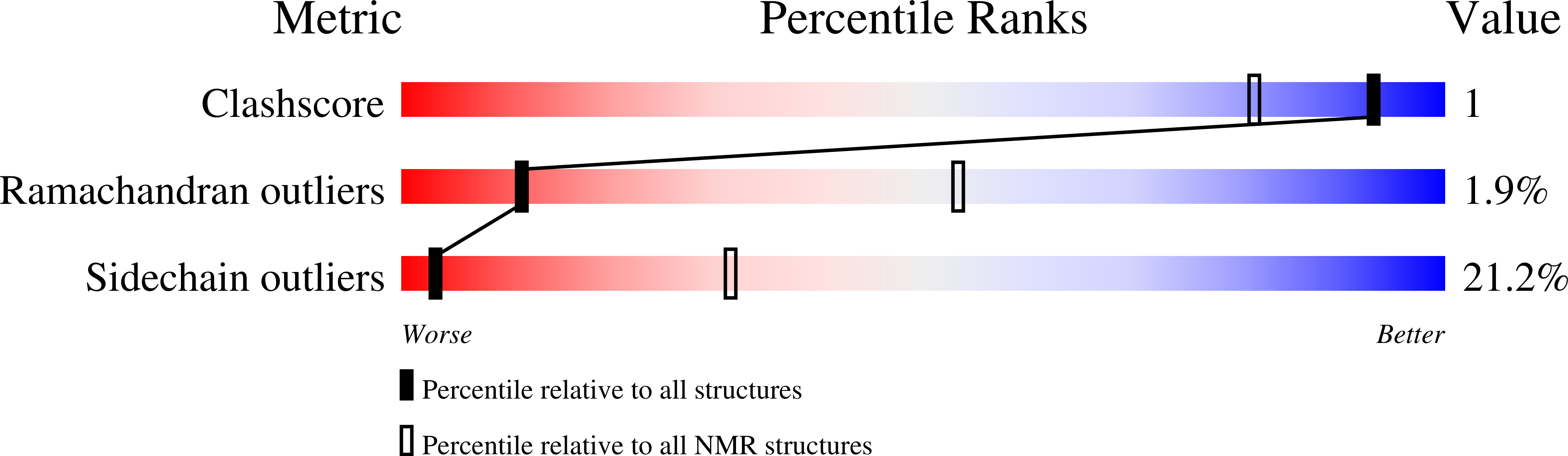Genome-wide and structural analysis of the Myb-SHAQKYF family in Entamoeba histolytica.
Cardenas-Hernandez, H., Titaux-Delgado, G.A., Castaneda-Ortiz, E.J., Torres-Larios, A., Brieba, L.G., Del Rio-Portilla, F., Azuara-Liceaga, E.(2021) Biochim Biophys Acta Proteins Proteom 1869: 140601-140601
- PubMed: 33422669
- DOI: https://doi.org/10.1016/j.bbapap.2021.140601
- Primary Citation of Related Structures:
6NVZ - PubMed Abstract:
Amoebiasis is the third leading cause of death among protozoon parasitic diseases in the lower-middle income countries. Understanding the molecular events that control gene expression such as transcription factors, their DNA binding mode and target sequences can help to develop new antiamoebic drugs against Entamoeba histolytica. In this paper we performed a genome and structural analysis of a specific transcription factor. The genome of E. histolytica codifies for 9 EhMybSHAQKYF proteins, which are a family within a large group of 34 Myb-DNA-binding domain (Myb-DBD) containing proteins. Here we compared Entamoeba Myb-SHAQKYF proteins with Myb-like proteins from the Reveille (RVE) family, important regulators of plant circadian networks. This comparison could lead to stablish their role in E. histolytica life cycle. We show that the ehmybshaqkyf genes are differentially expressed in trophozoites under basal cell culture conditions. An in-silico analysis predicts that members of this group harbor a highly conserved and structured Myb-DBD and a large portion of intrinsically disordered residues. As the Myb-DBD of these proteins harbors a distinctive Q[VI]R[ST]HAQK[YF]F sequence in its putative third α-helix, we consider relevant to determine the three-dimensional (3D) structure of one of them. An NMR structure of the Myb-DBD of EhMybS3 shows that this protein is composed of three α-helices stabilized by a hydrophobic core, similar to Myb proteins of different kingdoms. It is remarkable that despite not sharing similarities in their amino acid sequences, the structure of the Myb-DBD of the EhMybS3 is well conserved in this early branching eukaryote.
Organizational Affiliation:
Posgrado en Ciencias Genómicas, Universidad Autónoma de la Ciudad de México, Ciudad de México, México.