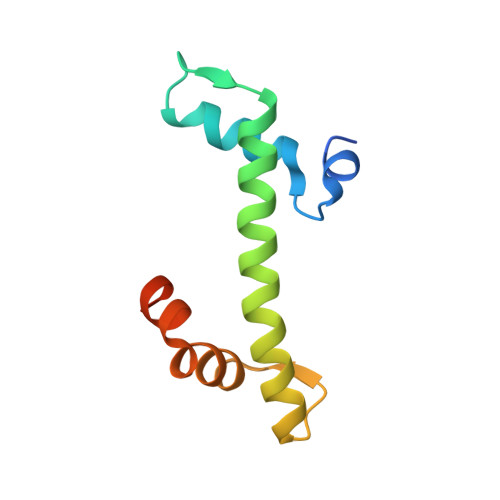
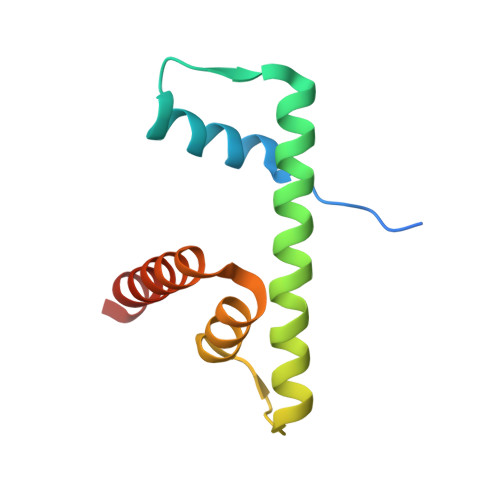
Role of a DEF/Y motif in histone H2A-H2B recognition and nucleosome editing.
Huang, Y., Sun, L., Pierrakeas, L., Dai, L., Pan, L., Luk, E., Zhou, Z.(2020) Proc Natl Acad Sci U S A 117: 3543-3550
- PubMed: 32001508
- DOI: https://doi.org/10.1073/pnas.1914313117
- Primary Citation of Related Structures:
6KBB - PubMed Abstract:
The SWR complex edits the histone composition of nucleosomes at promoters to facilitate transcription by replacing the two nucleosomal H2A-H2B (A-B) dimers with H2A.Z-H2B (Z-B) dimers. Swc5, a subunit of SWR, binds to A-B dimers, but its role in the histone replacement reaction was unclear. In this study, we showed that Swc5 uses a tandem DEF/Y motif within an intrinsically disordered region to engage the A-B dimer. A 2.37-Å X-ray crystal structure of the histone binding domain of Swc5 in complex with an A-B dimer showed that consecutive acidic residues and flanking hydrophobic residues of Swc5 form a cap over the histones, excluding histone-DNA interaction. Mutations in Swc5 DEF/Y inhibited the nucleosome editing function of SWR in vitro. Swc5 DEF/Y interacts with histones in vivo, and the extent of this interaction is dependent on the remodeling ATPase of SWR, supporting a model in which Swc5 acts as a wedge to promote A-B dimer eviction. Given that DEF/Y motifs are found in other evolutionary unrelated chromatin regulators, this work provides the molecular basis for a general strategy used repeatedly during eukaryotic evolution to mobilize histones in various genomic functions.
Organizational Affiliation:
National Laboratory of Biomacromolecules, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, 100101 Beijing, China.