Directed evolution of a genetically encoded immobilized lipase for the efficient production of biodiesel from waste cooking oil.
Heater, B.S., Chan, W.S., Lee, M.M., Chan, M.K.(2019) Biotechnol Biofuels 12: 165-165
- PubMed: 31297153
- DOI: https://doi.org/10.1186/s13068-019-1509-5
- Primary Citation of Related Structures:
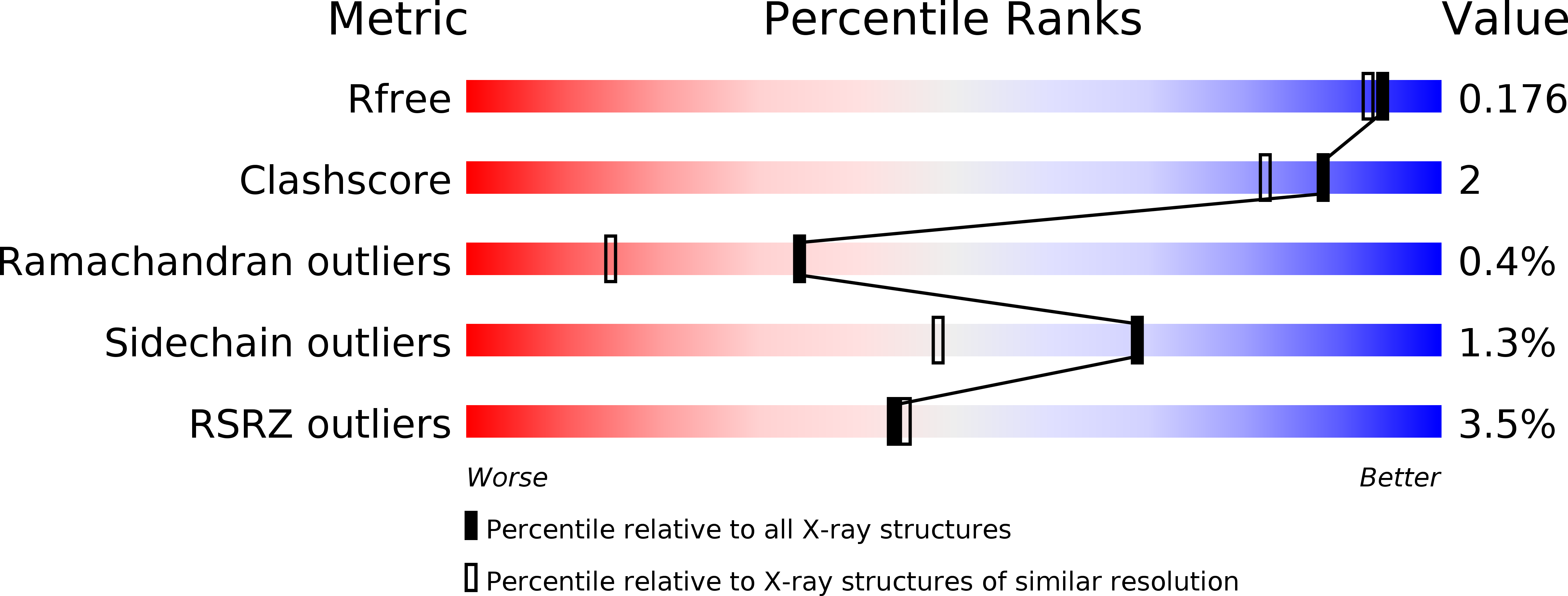
6JD9 - PubMed Abstract:
We have recently developed a one-step, genetically encoded immobilization approach based on fusion of a target enzyme to the self-crystallizing protein Cry3Aa, followed by direct production and isolation of the fusion crystals from Bacillus thuringiensis . Using this approach, Bacillus subtilis lipase A was genetically fused to Cry3Aa to produce a Cry3Aa-lipA catalyst capable of the facile conversion of coconut oil into biodiesel over 10 reaction cycles. Here, we investigate the fusion of another lipase to Cry3Aa with the goal of producing a catalyst suitable for the conversion of waste cooking oil into biodiesel. Genetic fusion of the Proteus mirabilis lipase (PML) to Cry3Aa allowed for the production of immobilized lipase crystals (Cry3Aa-PML) directly in bacterial cells. The fusion resulted in the loss of PML activity, however, and so taking advantage of its genetically encoded immobilization, directed evolution was performed on Cry3Aa-PML directly in its immobilized state in vivo. This novel strategy allowed for the selection of an immobilized PML mutant with 4.3-fold higher catalytic efficiency and improved stability. The resulting improved Cry3Aa-PML catalyst could be used to catalyze the conversion of waste cooking oil into biodiesel for at least 15 cycles with minimal loss in conversion efficiency. The genetically encoded nature of our Cry3Aa-fusion immobilization platform makes it possible to perform both directed evolution and screening of immobilized enzymes directly in vivo. This work is the first example of the use of directed evolution to optimize an enzyme in its immobilized state allowing for identification of a mutant that would unlikely have been identified from screening of its soluble form. We demonstrate that the resulting Cry3Aa-PML catalyst is suitable for the recyclable conversion of waste cooking oil into biodiesel.
Organizational Affiliation:
School of Life Sciences & Center of Novel Biomaterials, The Chinese University of Hong Kong, Hong Kong, SAR China.