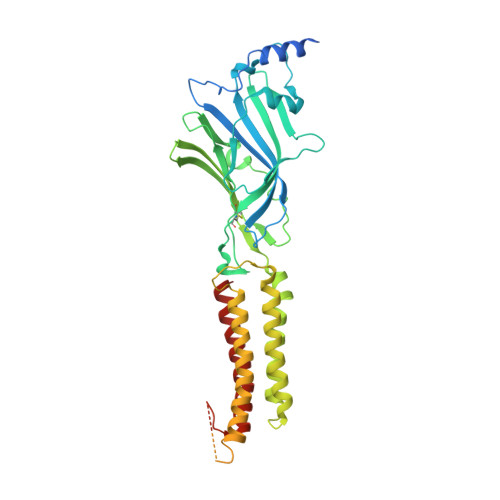
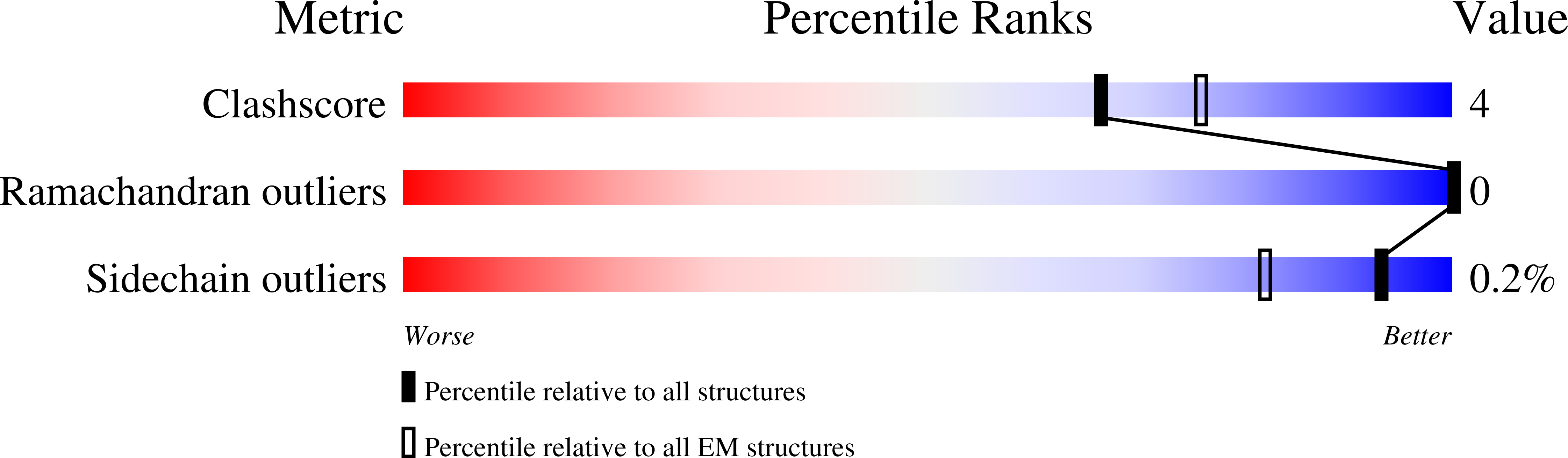Cryo-EM structure of the human alpha 1 beta 3 gamma 2 GABAAreceptor in a lipid bilayer.
Laverty, D., Desai, R., Uchanski, T., Masiulis, S., Stec, W.J., Malinauskas, T., Zivanov, J., Pardon, E., Steyaert, J., Miller, K.W., Aricescu, A.R.(2019) Nature 565: 516-520
- PubMed: 30602789
- DOI: https://doi.org/10.1038/s41586-018-0833-4
- Primary Citation of Related Structures:
6I53 - PubMed Abstract:
Type A γ-aminobutyric acid (GABA A ) receptors are pentameric ligand-gated ion channels and the main drivers of fast inhibitory neurotransmission in the vertebrate nervous system 1,2 . Their dysfunction is implicated in a range of neurological disorders, including depression, epilepsy and schizophrenia 3,4 . Among the numerous assemblies that are theoretically possible, the most prevalent in the brain are the α1β2/3γ2 GABA A receptors 5 . The β3 subunit has an important role in maintaining inhibitory tone, and the expression of this subunit alone is sufficient to rescue inhibitory synaptic transmission in β1-β3 triple knockout neurons 6 . So far, efforts to generate accurate structural models for heteromeric GABA A receptors have been hampered by the use of engineered receptors and the presence of detergents 7-9 . Notably, some recent cryo-electron microscopy reconstructions have reported 'collapsed' conformations 8,9 ; however, these disagree with the structure of the prototypical pentameric ligand-gated ion channel the Torpedo nicotinic acetylcholine receptor 10,11 , the large body of structural work on homologous homopentameric receptor variants 12 and the logic of an ion-channel architecture. Here we present a high-resolution cryo-electron microscopy structure of the full-length human α1β3γ2L-a major synaptic GABA A receptor isoform-that is functionally reconstituted in lipid nanodiscs. The receptor is bound to a positive allosteric modulator 'megabody' and is in a desensitized conformation. Each GABA A receptor pentamer contains two phosphatidylinositol-4,5-bisphosphate molecules, the head groups of which occupy positively charged pockets in the intracellular juxtamembrane regions of α1 subunits. Beyond this level, the intracellular M3-M4 loops are largely disordered, possibly because interacting post-synaptic proteins are not present. This structure illustrates the molecular principles of heteromeric GABA A receptor organization and provides a reference framework for future mechanistic investigations of GABAergic signalling and pharmacology.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Cambridge Biomedical Campus, Cambridge, UK. dlaverty@mrc-lmb.cam.ac.uk.