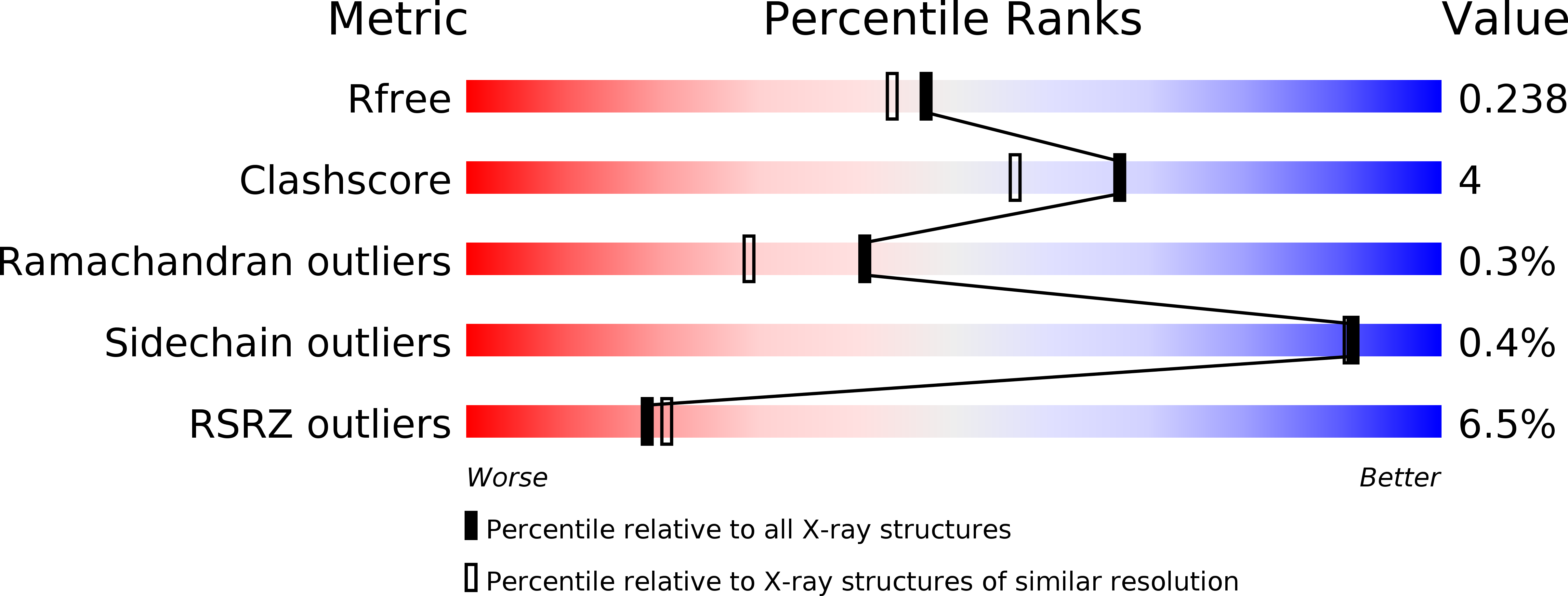
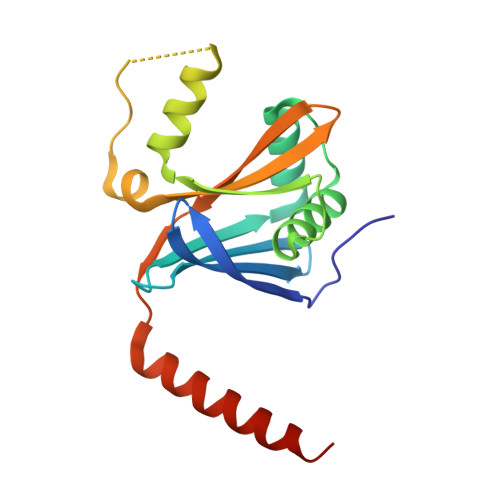
CCDC61/VFL3 Is a Paralog of SAS6 and Promotes Ciliary Functions.
Ochi, T., Quarantotti, V., Lin, H., Jullien, J., Rosa E Silva, I., Boselli, F., Barnabas, D.D., Johnson, C.M., McLaughlin, S.H., Freund, S.M.V., Blackford, A.N., Kimata, Y., Goldstein, R.E., Jackson, S.P., Blundell, T.L., Dutcher, S.K., Gergely, F., van Breugel, M.(2020) Structure 28: 674
- PubMed: 32375023
- DOI: https://doi.org/10.1016/j.str.2020.04.010
- Primary Citation of Related Structures:
6HXT, 6HXV, 6HXY - PubMed Abstract:
Centrioles are cylindrical assemblies whose peripheral microtubule array displays a 9-fold rotational symmetry that is established by the scaffolding protein SAS6. Centriole symmetry can be broken by centriole-associated structures, such as the striated fibers in Chlamydomonas that are important for ciliary function. The conserved protein CCDC61/VFL3 is involved in this process, but its exact role is unclear. Here, we show that CCDC61 is a paralog of SAS6. Crystal structures of CCDC61 demonstrate that it contains two homodimerization interfaces that are similar to those found in SAS6, but result in the formation of linear filaments rather than rings. Furthermore, we show that CCDC61 binds microtubules and that residues involved in CCDC61 microtubule binding are important for ciliary function in Chlamydomonas. Together, our findings suggest that CCDC61 and SAS6 functionally diverged from a common ancestor while retaining the ability to scaffold the assembly of basal body-associated structures or centrioles, respectively.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Cambridge Biomedical Campus, Francis Crick Avenue, Cambridge CB2 0QH, UK. Electronic address: T.Ochi@leeds.ac.uk.