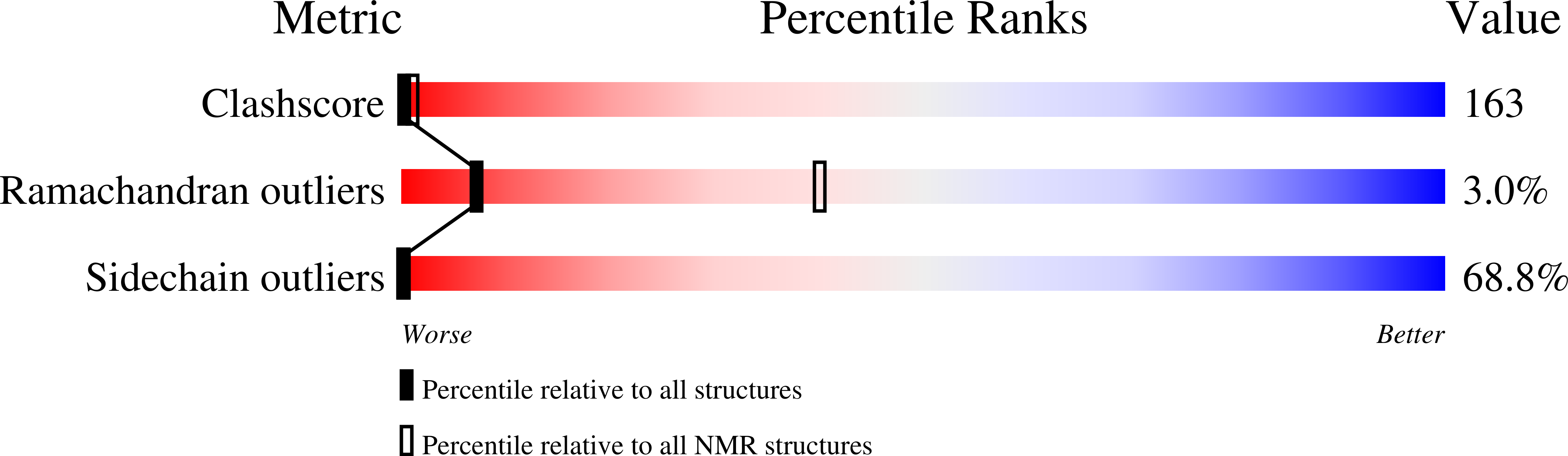A cation-pi interaction in a transmembrane helix of vacuolar ATPase retains the proton-transporting arginine in a hydrophobic environment.
Hohlweg, W., Wagner, G.E., Hofbauer, H.F., Sarkleti, F., Setz, M., Gubensak, N., Lichtenegger, S., Falsone, S.F., Wolinski, H., Kosol, S., Oostenbrink, C., Kohlwein, S.D., Zangger, K.(2018) J Biol Chem 293: 18977-18988
- PubMed: 30209131
- DOI: https://doi.org/10.1074/jbc.RA118.005276
- Primary Citation of Related Structures:
6HH0 - PubMed Abstract:
Vacuolar ATPases are multisubunit protein complexes that are indispensable for acidification and pH homeostasis in a variety of physiological processes in all eukaryotic cells. An arginine residue (Arg 735 ) in transmembrane helix 7 (TM7) of subunit a of the yeast ATPase is known to be essential for proton translocation. However, the specific mechanism of its involvement in proton transport remains to be determined. Arginine residues are usually assumed to "snorkel" toward the protein surface when exposed to a hydrophobic environment. Here, using solution NMR spectroscopy, molecular dynamics simulations, and in vivo yeast assays, we obtained evidence for the formation of a transient, membrane-embedded cation-π interaction in TM7 between Arg 735 and two highly conserved nearby aromatic residues, Tyr 733 and Trp 737 We propose a mechanism by which the transient, membrane-embedded cation-π complex provides the necessary energy to keep the charged side chain of Arg 735 within the hydrophobic membrane. Such cation-π interactions may define a general mechanism to retain charged amino acids in a hydrophobic membrane environment.
Organizational Affiliation:
From the Institutes of Chemistry and.