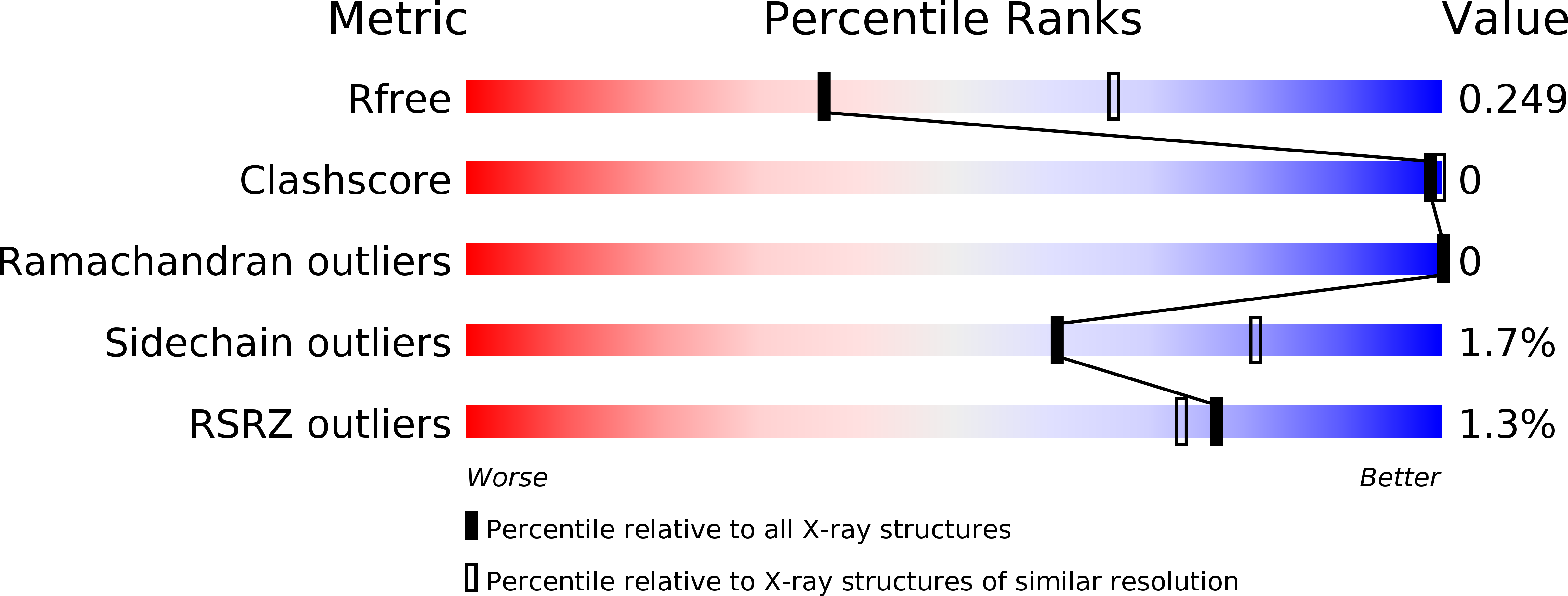
Unusual substrate and halide versatility of phenolic halogenase PltM.
Mori, S., Pang, A.H., Thamban Chandrika, N., Garneau-Tsodikova, S., Tsodikov, O.V.(2019) Nat Commun 10: 1255-1255
- PubMed: 30890712
- DOI: https://doi.org/10.1038/s41467-019-09215-9
- Primary Citation of Related Structures:
6BZA, 6BZI, 6BZN, 6BZQ, 6BZT, 6BZZ - PubMed Abstract:
Controlled halogenation of chemically versatile substrates is difficult to achieve. Here we describe a unique flavin-dependent halogenase, PltM, which is capable of utilizing a wide range of halides for installation on a diverse array of phenolic compounds, including FDA-approved drugs and natural products, such as terbutaline, fenoterol, resveratrol, and catechin. Crystal structures of PltM in complex with phloroglucinol and FAD in different states yield insight into substrate recognition and the FAD recycling mechanism of this halogenase.
Organizational Affiliation:
Department of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, Lexington, KY, 40536-0596, USA.