Discovery and Development of a Series of Pyrazolo[3,4-d]pyridazinone Compounds as the Novel Covalent Fibroblast Growth Factor Receptor Inhibitors by the Rational Drug Design.
Wang, Y., Dai, Y., Wu, X., Li, F., Liu, B., Li, C., Liu, Q., Zhou, Y., Wang, B., Zhu, M., Cui, R., Tan, X., Xiong, Z., Liu, J., Tan, M., Xu, Y., Geng, M., Jiang, H., Liu, H., Ai, J., Zheng, M.(2019) J Med Chem 62: 7473-7488
- PubMed: 31335138
- DOI: https://doi.org/10.1021/acs.jmedchem.9b00510
- Primary Citation of Related Structures:
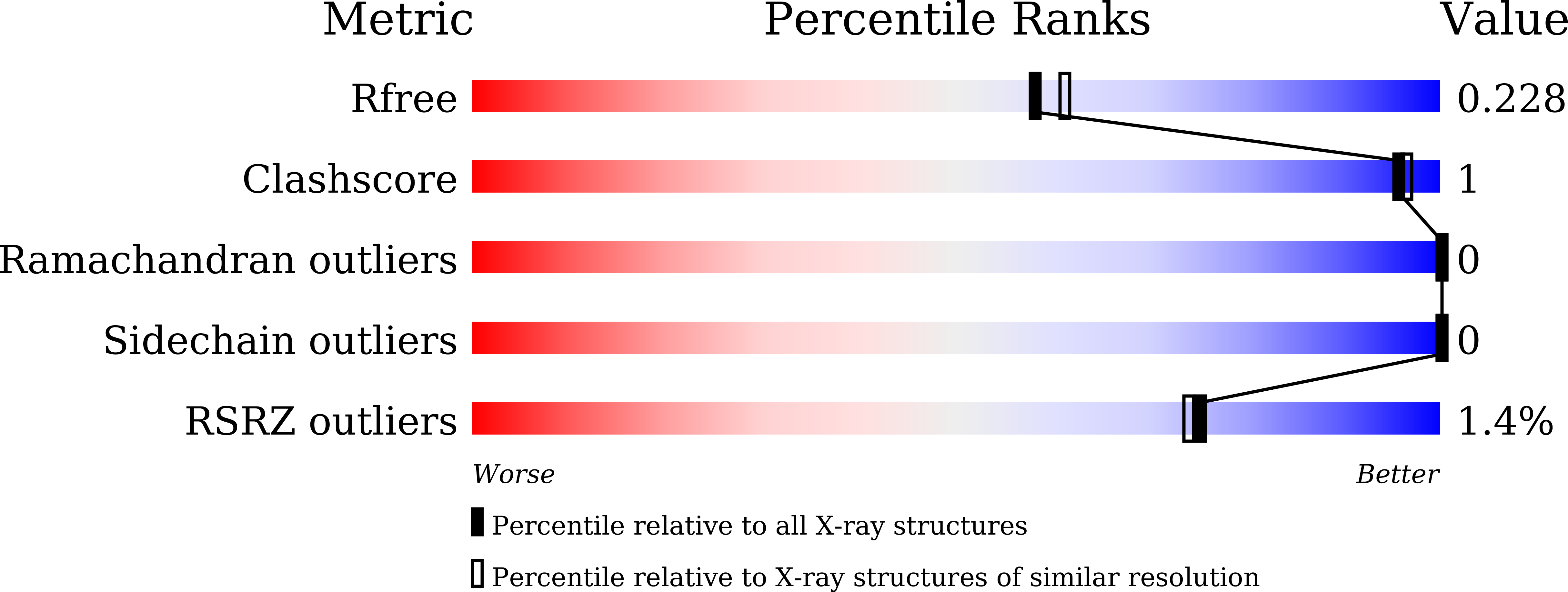
6ITJ, 6IUO, 6IUP - PubMed Abstract:
Alterations of fibroblast growth factor receptors (FGFRs) play key roles in numerous cancer progression and development, which makes FGFRs attractive targets in the cancer therapy. In the present study, based on a newly devised FGFR target-specific scoring function, a novel FGFR inhibitor hit was identified through virtual screening. Hit-to-lead optimization was then performed by integrating molecular docking and site-of-metabolism predictions with an array of in vitro evaluations and X-ray cocrystal structure determination, leading to a covalent FGFR inhibitor 15 , which showed a highly selective and potent FGFR inhibition profile. Pharmacokinetic assessment, protein kinase profiling, and hERG inhibition evaluation were also conducted, and they confirmed the value of 15 as a lead for further investigation. Overall, this study exemplifies the importance of the integrative use of computational methods and experimental techniques in drug discovery.
Organizational Affiliation:
School of Chemistry , Shanghai University , 99 ShangDa Road , Shanghai 200444 , China.