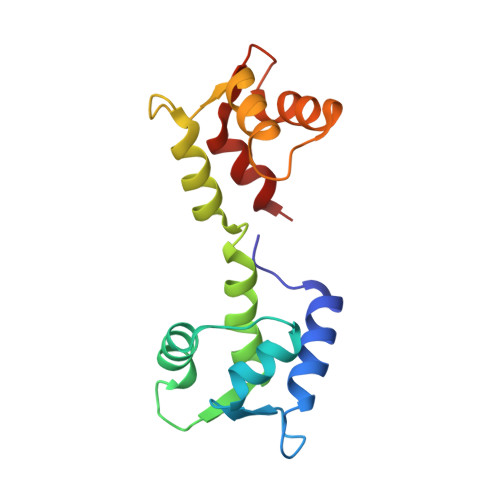
Na V 1.2 EFL domain allosterically enhances Ca 2+ binding to sites I and II of WT and pathogenic calmodulin mutants bound to the channel CTD.
Mahling, R., Hovey, L., Isbell, H.M., Marx, D.C., Miller, M.S., Kilpatrick, A.M., Weaver, L.D., Yoder, J.B., Kim, E.H., Andresen, C.N.J., Li, S., Shea, M.A.(2021) Structure
- PubMed: 33770503
- DOI: https://doi.org/10.1016/j.str.2021.03.002
- Primary Citation of Related Structures:
6BUT - PubMed Abstract:
Neuronal voltage-gated sodium channel Na V 1.2 C-terminal domain (CTD) binds calmodulin (CaM) constitutively at its IQ motif. A solution structure (6BUT) and other NMR evidence showed that the CaM N domain (CaM N ) is structurally independent of the C-domain (CaM C ) whether CaM is bound to the Na V 1.2 IQp (1,901-1,927) or Na V 1.2 CTD (1,777-1,937) with or without calcium. However, in the CaM + Na V 1.2 CTD complex, the Ca 2+ affinity of CaM N was more favorable than in free CaM, while Ca 2+ affinity for CaM C was weaker than in the CaM + Na V 1.2 IQp complex. The CTD EF-like (EFL) domain allosterically widened the energetic gap between CaM domains. Cardiomyopathy-associated CaM mutants (N53I(N54I), D95V(D96V), A102V(A103V), E104A(E105A), D129G(D130G), and F141L(F142L)) all bound the Na V 1.2 IQ motif favorably under resting (apo) conditions and bound calcium normally at CaM N sites. However, only N53I and A102V bound calcium at CaM C sites at [Ca 2+ ] < 100 μM. Thus, they are expected to respond like wild-type CaM to Ca 2+ spikes in excitable cells.
Organizational Affiliation:
Department of Biochemistry, Carver College of Medicine, University of Iowa, Iowa City, IA 52242-1109, USA.