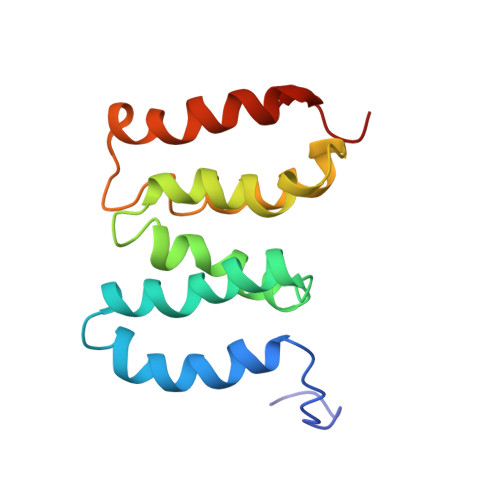
Solution structure of the N-terminal domain of proteasome lid subunit Rpn5
Zhang, W., Zhao, C., Hu, Y., Jin, C.(2018) Biochem Biophys Res Commun 504: 225-230
- PubMed: 30177392
- DOI: https://doi.org/10.1016/j.bbrc.2018.08.159
- Primary Citation of Related Structures:
5ZMR - PubMed Abstract:
The 26S proteasome is the major protein degradation machinery in living cells. The Rpn5 protein is one scaffolding subunit in the lid subcomplex of the 19S regulatory particle in the proteasome holoenzyme. Herein we report the solution structure of the N-terminal domain (NTD) of yeast Rpn5 at high resolution by NMR spectroscopy. The results show that Rpn5 NTD adopts α-solenoid-like fold in right-handed superhelical configuration formed by a number of α-helices. Structural comparisons with currently available cryo-EM structures reveal local structural differences in the first three helices between yeast and human Rpn5. The results further highlight the conformational flexibility in three possible protein interaction sites. Moreover, the structures of the NTD show large variations among different PCI-containing Rpn subunits. Our current results provide atomic-level structural basis for further investigations of protein-protein interactions and the proteasome assembly pathway.
Organizational Affiliation:
College of Life Sciences, Peking University, Beijing 100871, China; Beijing Nuclear Magnetic Resonance Center, Peking University, Beijing 100871, China.