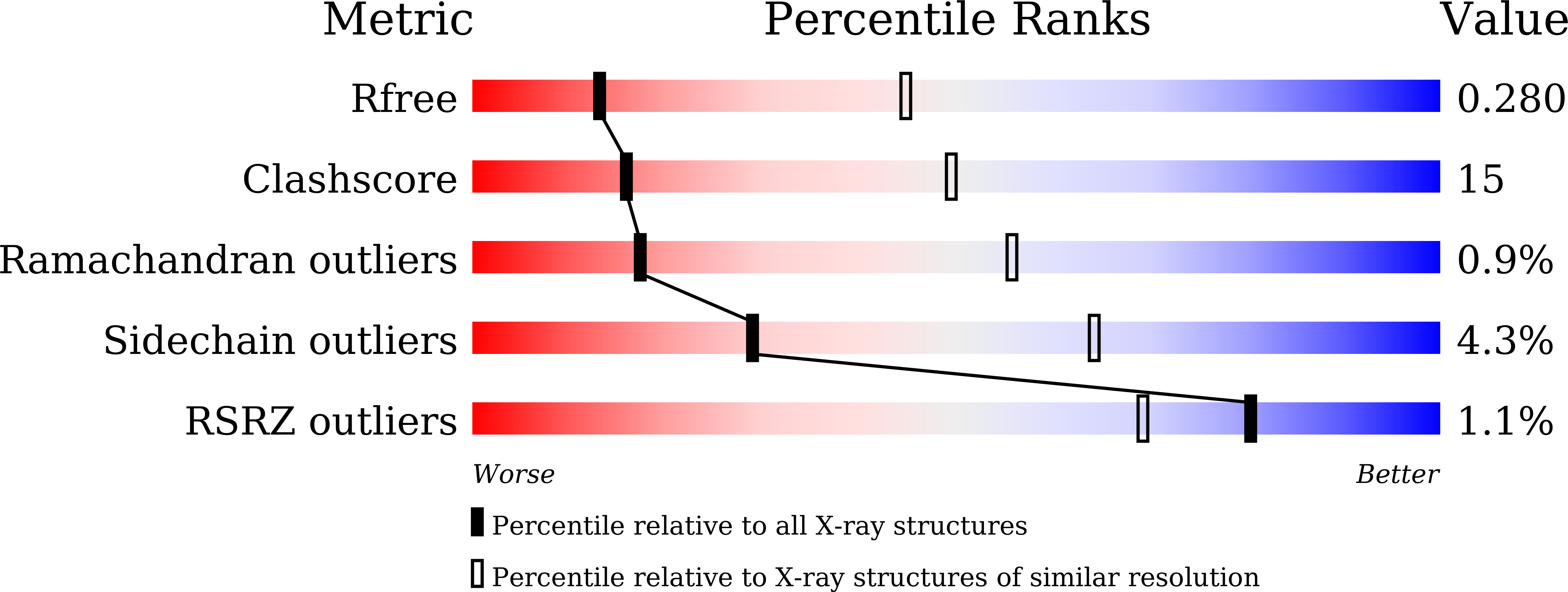
Structure of the enterovirus D68 RNA-dependent RNA polymerase in complex with NADPH implicates an inhibitor binding site in the RNA template tunnel.
Li, L., Wang, M., Chen, Y., Hu, T., Yang, Y., Zhang, Y., Bi, G., Wang, W., Liu, E., Han, J., Lu, T., Su, D.(2020) J Struct Biol : 107510-107510
- PubMed: 32353513
- DOI: https://doi.org/10.1016/j.jsb.2020.107510
- Primary Citation of Related Structures:
5ZIT, 6L4R - PubMed Abstract:
Enterovirus D68 (EV-D68) is an emerging viral pathogen belonging to the Enterovirus genus of the Picornaviridae family, which is a serious threat to human health and has resulted in significant economic losses. The EV-D68 genome encodes an RNA-dependent RNA polymerase (RdRp) 3D pol , which is central for viral genome replication and considered as a promising target for specific antiviral therapeutics. In this study, we report the crystal structures of human EV-D68 RdRp in the apo state and in complex with the inhibitor NADPH, which was selected by using a structure-based virtual screening approach. The EV-D68-RdRp-NADPH complex is the first RdRp-inhibitor structure identified in the species Enterovirus D. The inhibitor NADPH occupies the RNA template binding channel of EV-D68 RdRp with a novel binding pocket. Additionally, residues involved in the NADPH binding pocket of EV-D68 RdRp are highly conserved in RdRps of enteroviruses. Therefore, the enzyme activity of three RdRps from EV-D68, poliovirus, and enterovirus A71 is shown to decrease when titrated with NADPH separately in vitro. Furthermore, we identified that NADPH plays a pivotal role as an RdRp inhibitor instead of a chain terminator during restriction of RNA-dependent RNA replication. In the future, derivatives of NADPH may pave the way for novel inhibitors of RdRp through compound modification, providing potential antiviral agents for treating enteroviral infection and related diseases.
Organizational Affiliation:
State Key Lab of Biotherapy and Cancer Center, West China Hospital, Sichuan University and Collaborative Innovation Center for Biotherapy, Chengdu, China.