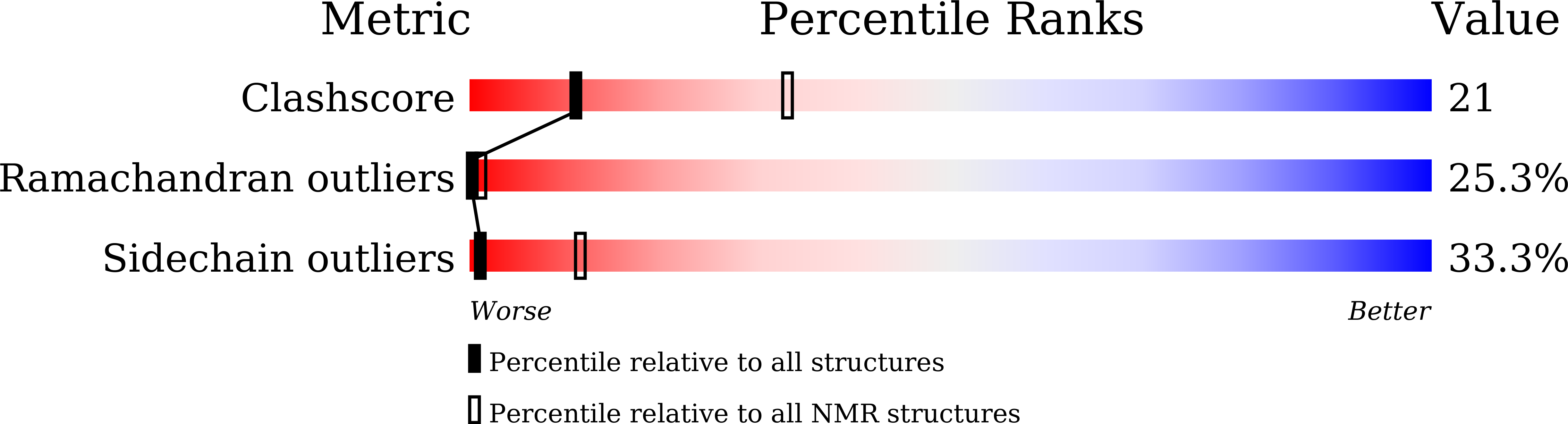Heterologous production of a new lasso peptide brevunsin in Sphingomonas subterranea
Kodani, S., Hemmi, H., Miyake, Y., Kaweewan, I., Nakagawa, H.(2018) J Ind Microbiol Biotechnol 45: 983-992
- PubMed: 30191430
- DOI: https://doi.org/10.1007/s10295-018-2077-6
- Primary Citation of Related Structures:
5ZCN - PubMed Abstract:
A shuttle vector pHSG396Sp was constructed to perform gene expression using Sphingomonas subterranea as a host. A new lasso peptide biosynthetic gene cluster, derived from Brevundimonas diminuta, was amplified by PCR and integrated to afford a expression vector pHSG396Sp-12697L. The new lasso peptide brevunsin was successfully produced by S. subterranea, harboring the expression vector, with a high production yield (10.2 mg from 1 L culture). The chemical structure of brevunsin was established by NMR and MS/MS experiments. Based on the information obtained from the NOE experiment, the three-dimensional structure of brevunsin was determined, which indicated that brevunsin possessed a typical lasso structure. This expression vector system provides a new heterologous production method for unexplored lasso peptides that are encoded by bacterial genomes.
Organizational Affiliation:
College of Agriculture, Academic Institute, Shizuoka University, 836 Ohya, Suruga-ku, Shizuoka, 422-8529, Japan. kodani.shinya@shizuoka.ac.jp.