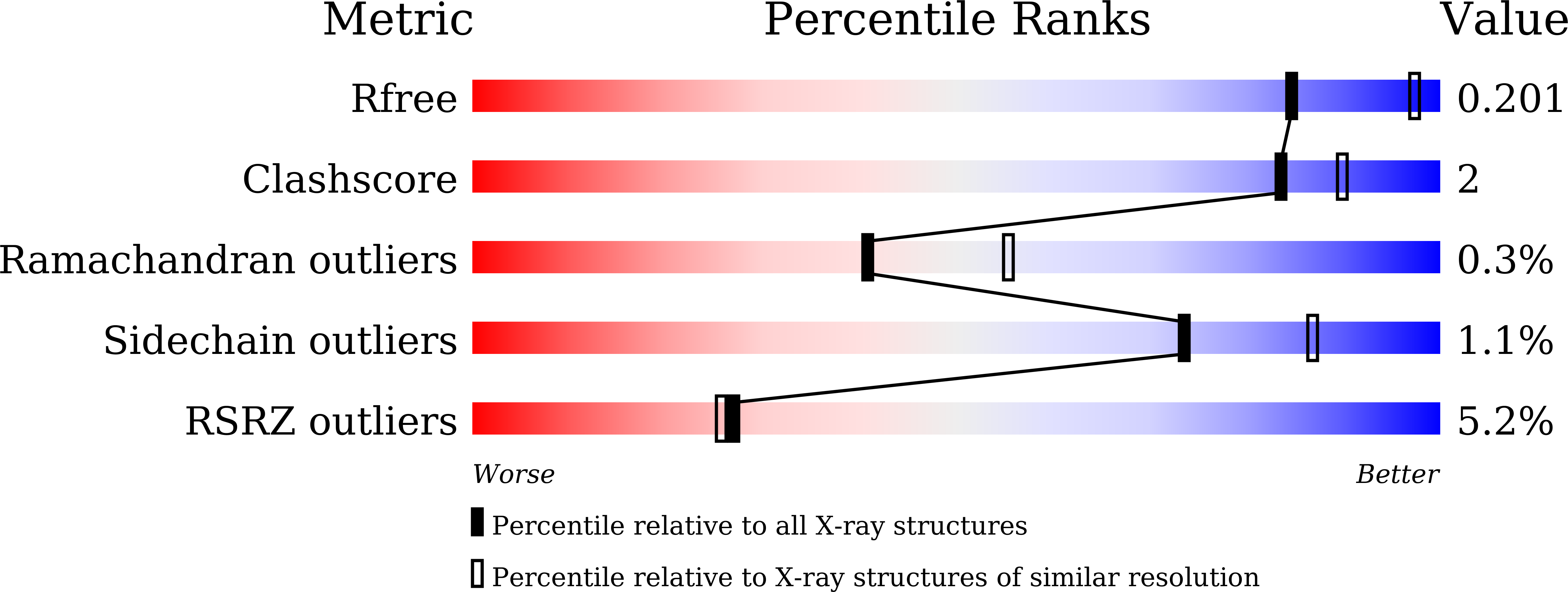
Molecular basis for histidine N1 position-specific methylation by CARNMT1.
Cao, R., Zhang, X., Liu, X., Li, Y., Li, H.(2018) Cell Res 28: 494-496
- PubMed: 29463897
- DOI: https://doi.org/10.1038/s41422-018-0003-0
- Primary Citation of Related Structures:
5YF0, 5YF1, 5YF2
Organizational Affiliation:
MOE Key Laboratory of Protein Sciences, Beijing Advanced Innovation Center for Structural Biology, Tsinghua-Peking Joint Center for Life Sciences, Department of Basic Medical Sciences, School of Medicine, Tsinghua University, Beijing, 100084, China.