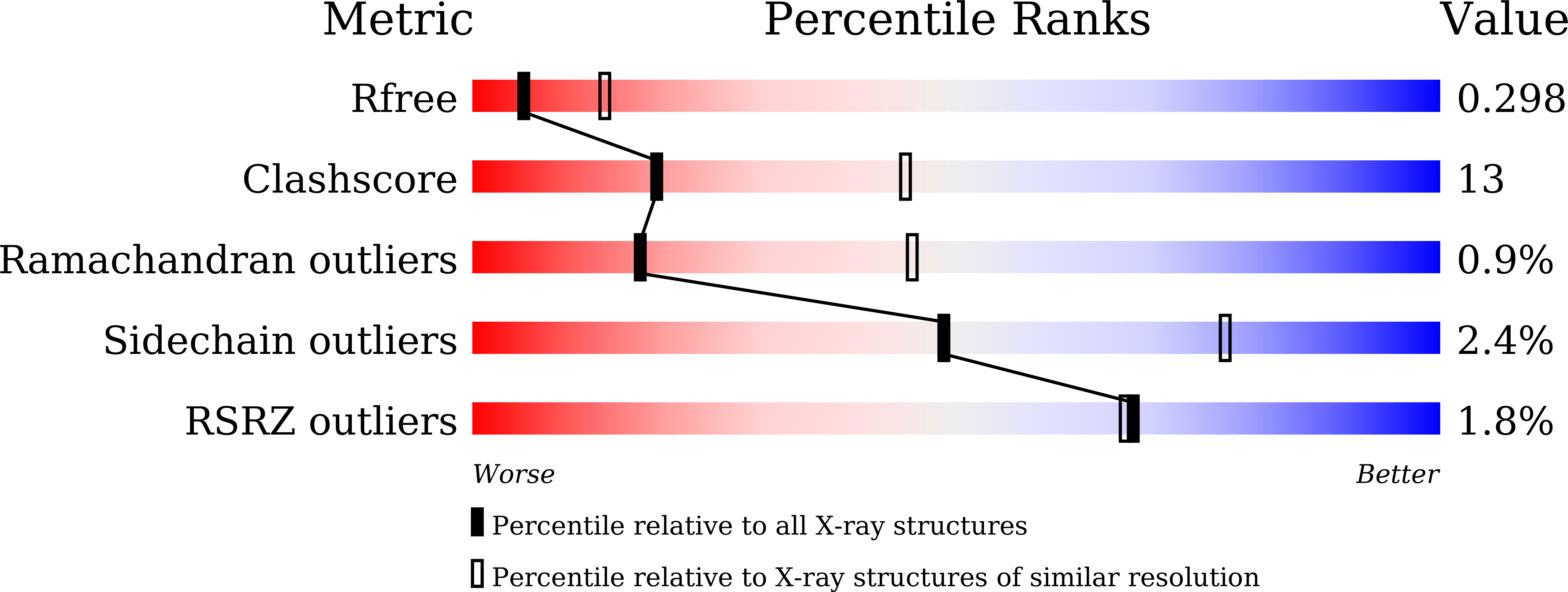
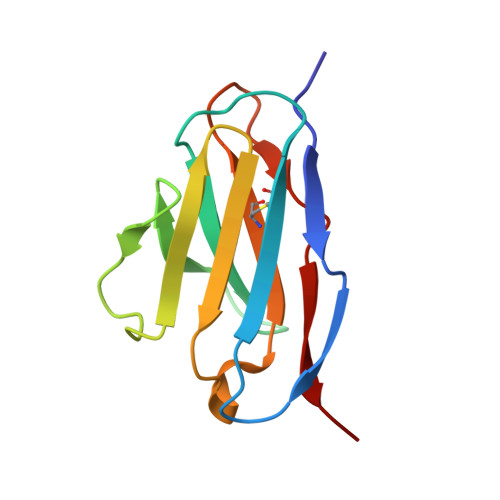
Heat-induced native dimerization prevents amyloid formation by variable domain from immunoglobulin light-chain REI
Nawata, M., Tsutsumi, H., Kobayashi, Y., Unzai, S., Mine, S., Nakamura, T., Uegaki, K., Kamikubo, H., Kataoka, M., Hamada, D.(2017) FEBS J 284: 3114-3127
- PubMed: 28736891
- DOI: https://doi.org/10.1111/febs.14181
- Primary Citation of Related Structures:
5XP1, 5XQY - PubMed Abstract:
Amyloid light-chain (AL) amyloidosis is a protein-misfolding disease characterized by accumulation of immunoglobulin light chains (LCs) into amyloid fibrils. Dimerization of a full length or variable domain (V L ) of LC serves to stabilize the native state and prevent the formation of amyloid fibrils. We here analyzed the thermodynamic properties of dimerization and unfolding reactions by nonamyloidogenic V L from REI LC or its monomeric Y96K mutant using sedimentation velocity and circular dichroism. The data indicate that the equilibrium shifts to native dimerization for wild-type REI V L by elevating temperature due to the negative enthalpy change for dimer dissociation (-81.2 kJ·mol -1 ). The Y96K mutation did not affect the stability of the monomeric native state but increased amyloidogenicity. These results suggest that the heat-induced native homodimerization is the major factor preventing amyloid formation by wild-type REI V L . Heat-induced native oligomerization may be an efficient strategy to avoid the formation of misfolded aggregates particularly for thermostable proteins that are used at elevated temperatures under conditions where other proteins tend to misfold.
Organizational Affiliation:
Graduate School of Medicine, Kobe University, Japan.