Mechanistic insight into how multidrug resistant Acinetobacter baumannii response regulator AdeR recognizes an intercistronic region.
Wen, Y., Ouyang, Z., Yu, Y., Zhou, X., Pei, Y., Devreese, B., Higgins, P.G., Zheng, F.(2017) Nucleic Acids Res 45: 9773-9787
- PubMed: 28934482
- DOI: https://doi.org/10.1093/nar/gkx624
- Primary Citation of Related Structures:
5X5J, 5X5L, 5XJP - PubMed Abstract:
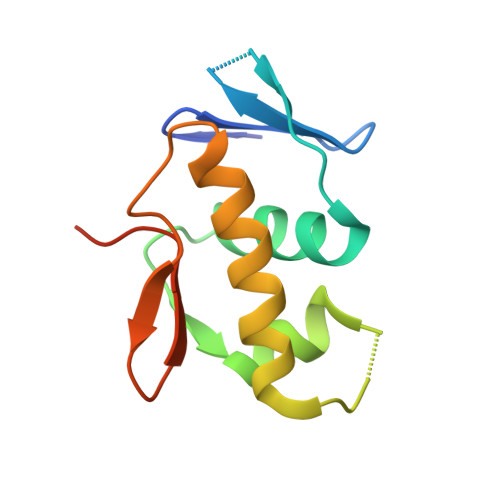
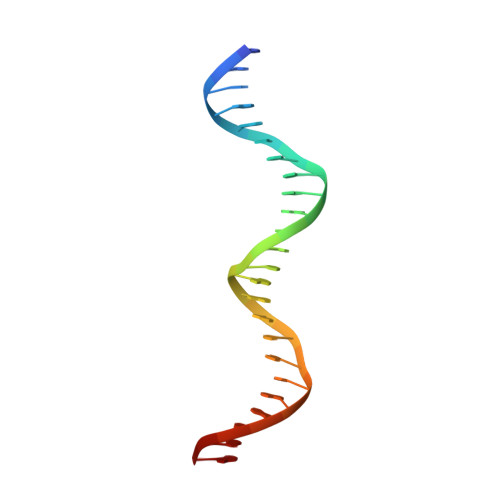
AdeR-AdeS is a two-component regulatory system, which controls expression of the adeABC efflux pump involved in Acinetobacter baumannii multidrug resistance. AdeR is a response regulator consisting of an N-terminal receiver domain and a C-terminal DNA-binding-domain. AdeR binds to a direct-repeat DNA in the intercistronic region between adeR and adeABC. We demonstrate a markedly high affinity binding between unphosphorylated AdeR and DNA with a dissociation constant of 20 nM. In addition, we provide a 2.75 Å crystal structure of AdeR DNA-binding-domain complexed with the intercistronic DNA. This structure shows that the α3 and β hairpin formed by β5-β6 interacts with the major and minor groove of the DNA, which in turn leads to the introduction of a bend. The AdeR receiver domain structure revealed a dimerization motif mediated by a gearwheel-like structure involving the D108F109-R122 motif through cation π stack interaction. The structure of AdeR receiver domain bound with magnesium indicated a conserved Glu19Asp20-Asp63 magnesium-binding motif, and revealed that the potential phosphorylation site Asp63OD1 forms a hydrogen bond with Lys112. We thus dissected the mechanism of how AdeR recognizes the intercistronic DNA, which leads to a diverse mode of response regulation. Unlocking the AdeRS mechanism provides ways to circumvent A. baumannii antibiotic resistance.
Organizational Affiliation:
Center for Translational Medicine, The Key Laboratory of Biomedical Information Engineering of Ministry of Education, School of Life Science and Technology, Xi'an Jiaotong University, Xi'an 710061, China.