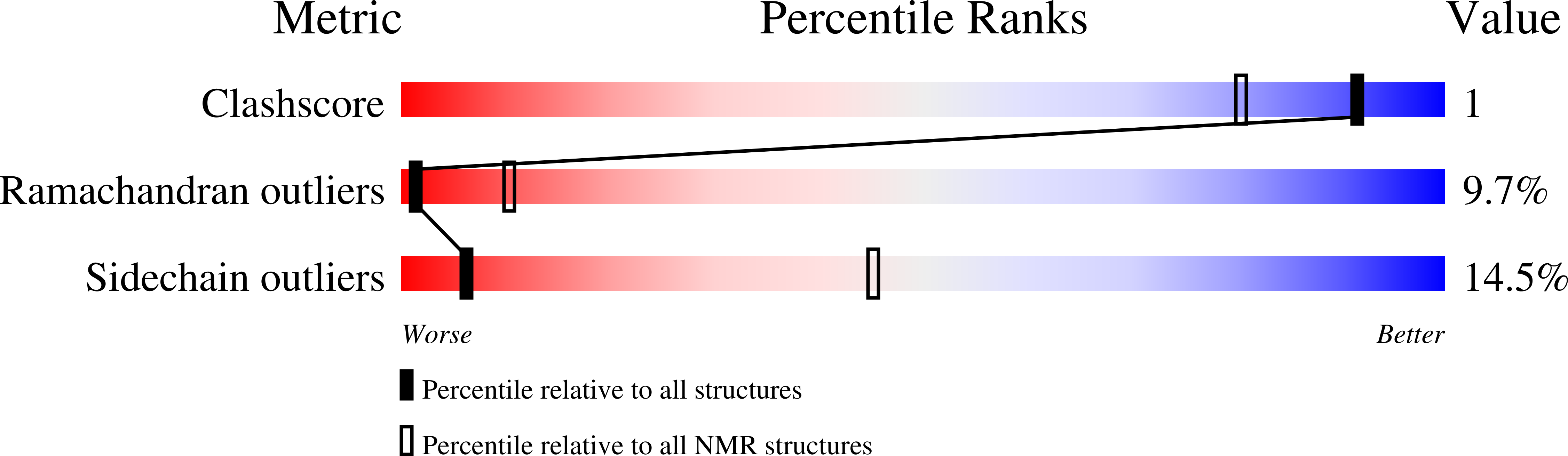Structure of FUS Protein Fibrils and Its Relevance to Self-Assembly and Phase Separation of Low-Complexity Domains.
Murray, D.T., Kato, M., Lin, Y., Thurber, K.R., Hung, I., McKnight, S.L., Tycko, R.(2017) Cell 171: 615-627.e16
- PubMed: 28942918
- DOI: https://doi.org/10.1016/j.cell.2017.08.048
- Primary Citation of Related Structures:
5W3N - PubMed Abstract:
Polymerization and phase separation of proteins containing low-complexity (LC) domains are important factors in gene expression, mRNA processing and trafficking, and localization of translation. We have used solid-state nuclear magnetic resonance methods to characterize the molecular structure of self-assembling fibrils formed by the LC domain of the fused in sarcoma (FUS) RNA-binding protein. From the 214-residue LC domain of FUS (FUS-LC), a segment of only 57 residues forms the fibril core, while other segments remain dynamically disordered. Unlike pathogenic amyloid fibrils, FUS-LC fibrils lack hydrophobic interactions within the core and are not polymorphic at the molecular structural level. Phosphorylation of core-forming residues by DNA-dependent protein kinase blocks binding of soluble FUS-LC to FUS-LC hydrogels and dissolves phase-separated, liquid-like FUS-LC droplets. These studies offer a structural basis for understanding LC domain self-assembly, phase separation, and regulation by post-translational modification.
Organizational Affiliation:
Laboratory of Chemical Physics, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892-0520, USA; Postdoctoral Research Associate Program, National Institute of General Medical Sciences, National Institutes of Health, Bethesda, MD 20892-6200, USA.