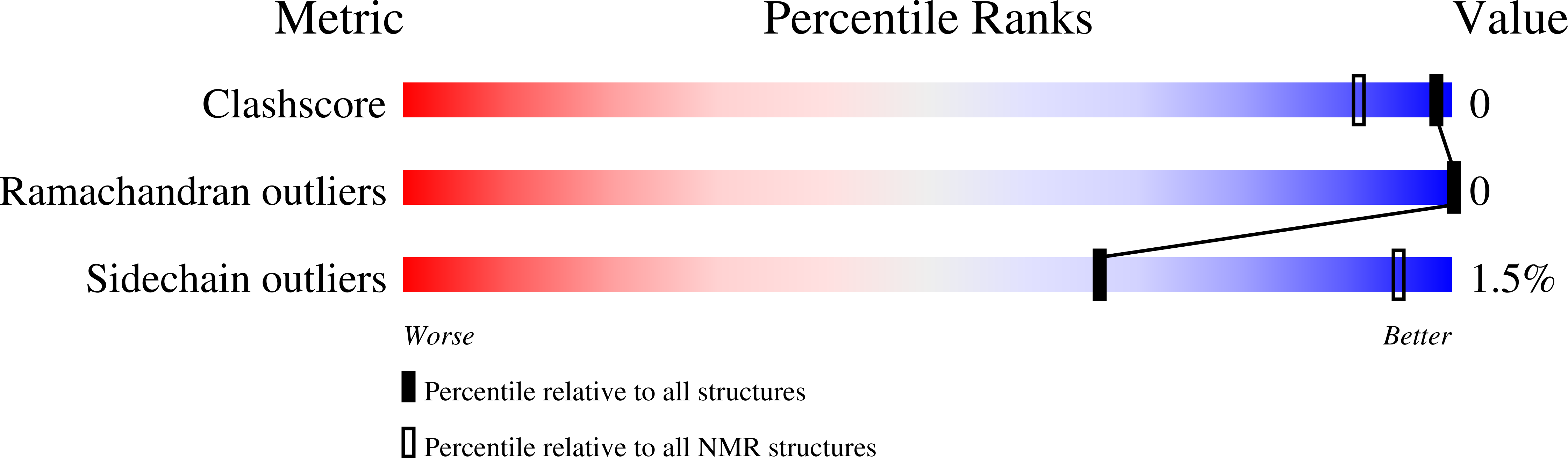NMR solution structure of the RED subdomain of the Sleeping Beauty transposase.
Konnova, T.A., Singer, C.M., Nesmelova, I.V.(2017) Protein Sci 26: 1171-1181
- PubMed: 28345263
- DOI: https://doi.org/10.1002/pro.3167
- Primary Citation of Related Structures:
5UNK - PubMed Abstract:
DNA transposons can be employed for stable gene transfer in vertebrates. The Sleeping Beauty (SB) DNA transposon has been recently adapted for human application and is being evaluated in clinical trials, however its molecular mechanism is not clear. SB transposition is catalyzed by the transposase enzyme, which is a multi-domain protein containing the catalytic and the DNA-binding domains. The DNA-binding domain of the SB transposase contains two structurally independent subdomains, PAI and RED. Recently, the structures of the catalytic domain and the PAI subdomain have been determined, however no structural information on the RED subdomain and its interactions with DNA has been available. Here, we used NMR spectroscopy to determine the solution structure of the RED subdomain and characterize its interactions with the transposon DNA.
Organizational Affiliation:
Department of Physics and Optical Science, University of North Carolina, Charlotte, North Carolina, 28223.