The ER stress sensor PERK luminal domain functions as a molecular chaperone to interact with misfolded proteins.
Wang, P., Li, J., Sha, B.(2016) Acta Crystallogr D Struct Biol 72: 1290-1297
- PubMed: 27917829
- DOI: https://doi.org/10.1107/S2059798316018064
- Primary Citation of Related Structures:
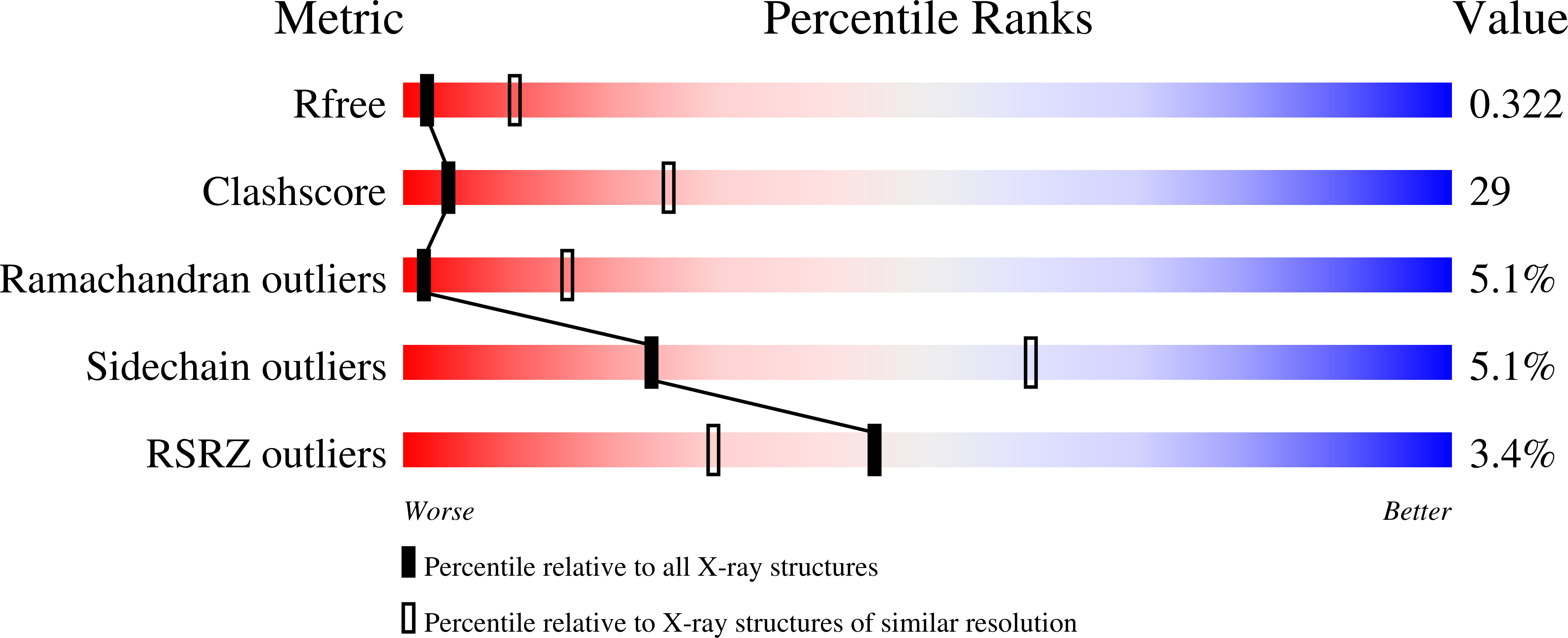
5SV7 - PubMed Abstract:
PERK is one of the major sensor proteins which can detect the protein-folding imbalance generated by endoplasmic reticulum (ER) stress. It remains unclear how the sensor protein PERK is activated by ER stress. It has been demonstrated that the PERK luminal domain can recognize and selectively interact with misfolded proteins but not native proteins. Moreover, the PERK luminal domain may function as a molecular chaperone to directly bind to and suppress the aggregation of a number of misfolded model proteins. The data strongly support the hypothesis that the PERK luminal domain can interact directly with misfolded proteins to induce ER stress signaling. To illustrate the mechanism by which the PERK luminal domain interacts with misfolded proteins, the crystal structure of the human PERK luminal domain was determined to 3.2 Å resolution. Two dimers of the PERK luminal domain constitute a tetramer in the asymmetric unit. Superimposition of the PERK luminal domain molecules indicated that the β-sandwich domain could adopt multiple conformations. It is hypothesized that the PERK luminal domain may utilize its flexible β-sandwich domain to recognize and interact with a broad range of misfolded proteins.
Organizational Affiliation:
Department of Cell, Developmental and Integrative Biology (CDIB), University of Alabama at Birmingham, Birmingham, AL 35294, USA.