Structure and function of a CE4 deacetylase isolated from a marine environment.
Tuveng, T.R., Rothweiler, U., Udatha, G., Vaaje-Kolstad, G., Smalas, A., Eijsink, V.G.H.(2017) PLoS One 12: e0187544-e0187544
- PubMed: 29107991
- DOI: https://doi.org/10.1371/journal.pone.0187544
- Primary Citation of Related Structures:
5LFZ, 5LGC - PubMed Abstract:
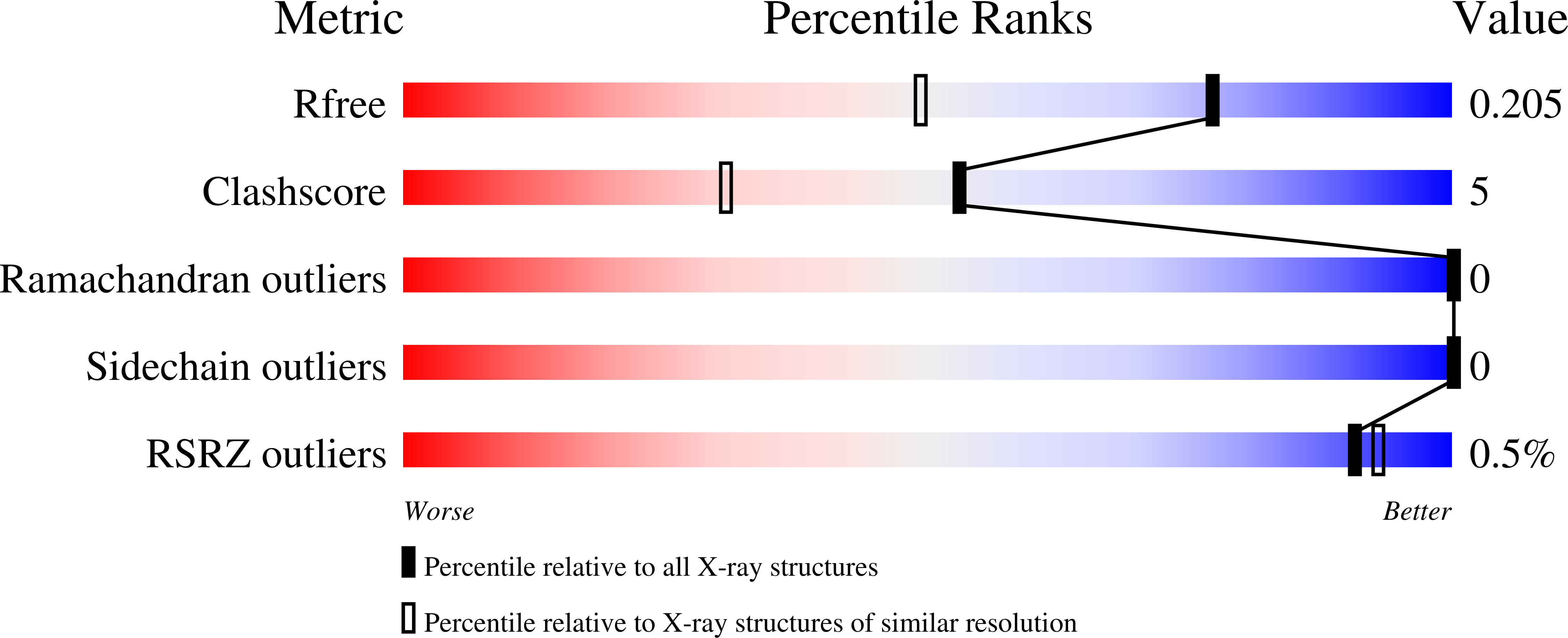
Chitin, a polymer of β(1-4)-linked N-acetylglucosamine found in e.g. arthropods, is a valuable resource that may be used to produce chitosan and chitooligosaccharides, two compounds with considerable industrial and biomedical potential. Deacetylating enzymes may be used to tailor the properties of chitin and its derived products. Here, we describe a novel CE4 enzyme originating from a marine Arthrobacter species (ArCE4A). Crystal structures of this novel deacetylase were determined, with and without bound chitobiose [(GlcNAc)2], and refined to 2.1 Å and 1.6 Å, respectively. In-depth biochemical characterization showed that ArCE4A has broad substrate specificity, with higher activity against longer oligosaccharides. Mass spectrometry-based sequencing of reaction products generated from a fully acetylated pentamer showed that internal sugars are more prone to deacetylation than the ends. These enzyme properties are discussed in the light of the structure of the enzyme-ligand complex, which adds valuable information to our still rather limited knowledge on enzyme-substrate interactions in the CE4 family.
Organizational Affiliation:
Faculty of Chemistry, Biotechnology and Food Science, Norwegian University of Life Sciences (NMBU), Ås, Norway.