Evolution of Protein Quaternary Structure in Response to Selective Pressure for Increased Thermostability.
Fraser, N.J., Liu, J.W., Mabbitt, P.D., Correy, G.J., Coppin, C.W., Lethier, M., Perugini, M.A., Murphy, J.M., Oakeshott, J.G., Weik, M., Jackson, C.J.(2016) J Mol Biol 428: 2359-2371
- PubMed: 27016206
- DOI: https://doi.org/10.1016/j.jmb.2016.03.014
- Primary Citation of Related Structures:
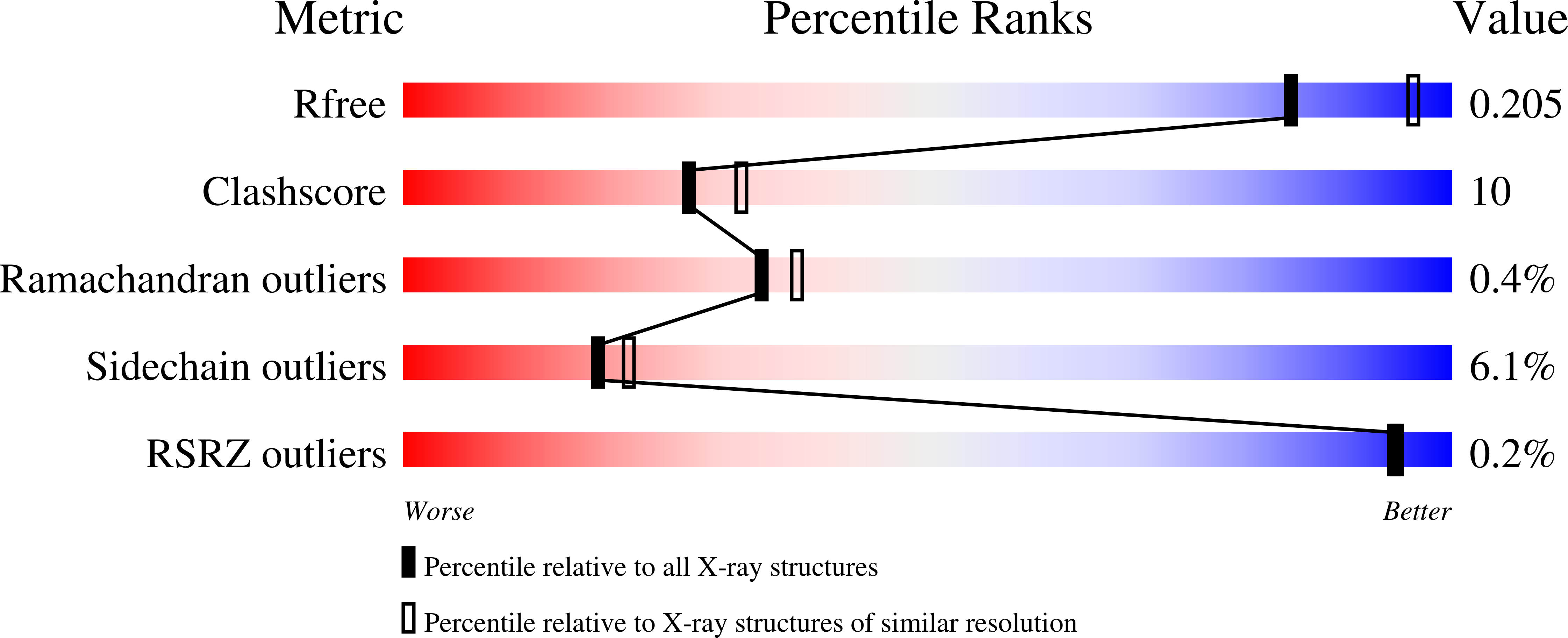
5IKX - PubMed Abstract:
Oligomerization has been suggested to be an important mechanism for increasing or maintaining the thermostability of proteins. Although it is evident that protein-protein contacts can result in substantial stabilization in many extant proteins, evidence for evolutionary selection for oligomerization is largely indirect and little is understood of the early steps in the evolution of oligomers. A laboratory-directed evolution experiment that selected for increased thermostability in the αE7 carboxylesterase from the Australian sheep blowfly, Lucilia cuprina, resulted in a thermostable variant, LcαE7-4a, that displayed increased levels of dimeric and tetrameric quaternary structure. A trade-off between activity and thermostability was made during the evolution of thermostability, with the higher-order oligomeric species displaying the greatest thermostability and lowest catalytic activity. Analysis of monomeric and dimeric LcαE7-4a crystal structures revealed that only one of the oligomerization-inducing mutations was located at a potential protein-protein interface. This work demonstrates that by imposing a selective pressure demanding greater thermostability, mutations can lead to increased oligomerization and stabilization, providing support for the hypothesis that oligomerization is a viable evolutionary strategy for protein stabilization.
Organizational Affiliation:
Research School of Chemistry, Australian National University, Canberra, ACT 2601, Australia.