Mechanism of TAp73 inhibition by Delta Np63 and structural basis of p63/p73 hetero-tetramerization.
Gebel, J., Luh, L.M., Coutandin, D., Osterburg, C., Lohr, F., Schafer, B., Frombach, A.S., Sumyk, M., Buchner, L., Krojer, T., Salah, E., Mathea, S., Guntert, P., Knapp, S., Dotsch, V.(2016) Cell Death Differ 23: 1930-1940
- PubMed: 27716744
- DOI: https://doi.org/10.1038/cdd.2016.83
- Primary Citation of Related Structures:
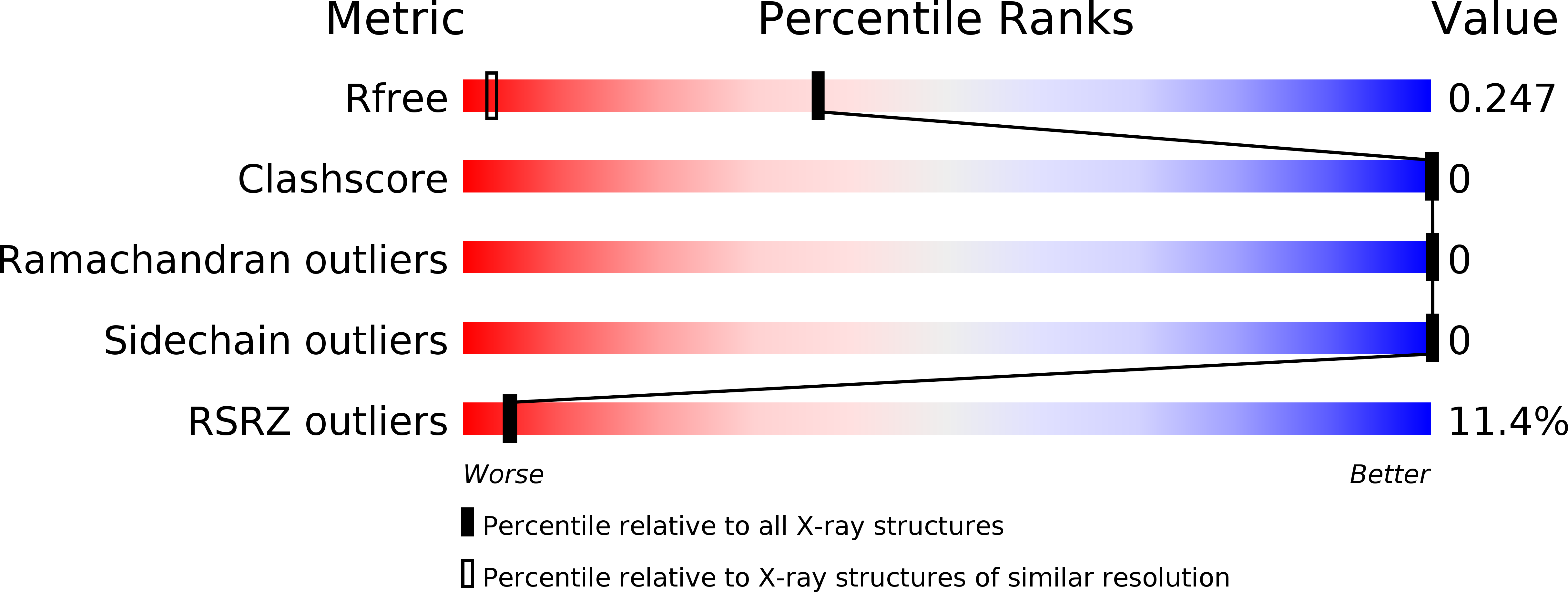
2NB1, 5HOC - PubMed Abstract:
Members of the p53 tumor-suppressor family are expressed as multiple isoforms. Isoforms with an N-terminal transactivation domain are transcriptionally active, while those ones lacking this domain often inhibit the transcriptional activity of other family members. In squamous cell carcinomas, the high expression level of ΔNp63α inhibits the tumor-suppressor function of TAp73β. This can in principle be due to blocking of the promoter or by direct interaction between both proteins. p63 and p73 can hetero-oligomerize through their tetramerization domains and a hetero-tetramer consisting of two p63 and two p73 molecules is thermodynamically more stable than both homo-tetramers. Here we show that cells expressing both p63 and p73 exist in mouse epidermis and hair follicle and that hetero-tetramer complexes can be detected by immunoprecipitation in differentiating keratinocytes. Through structure determination of the hetero-tetramer, we reveal why this hetero-tetramer is the thermodynamically preferred species. We have created mutants that exclusively form either hetero-tetramers or homo-tetramers, allowing to investigate the function of these p63/p73 hetero-tetramers. Using these tools, we show that inhibition of TAp73β in squamous cell carcinomas is due to promoter squelching and not direct interaction.
Organizational Affiliation:
Institute of Biophysical Chemistry and Center for Biomolecular Magnetic Resonance, Goethe University, Frankfurt, Germany.