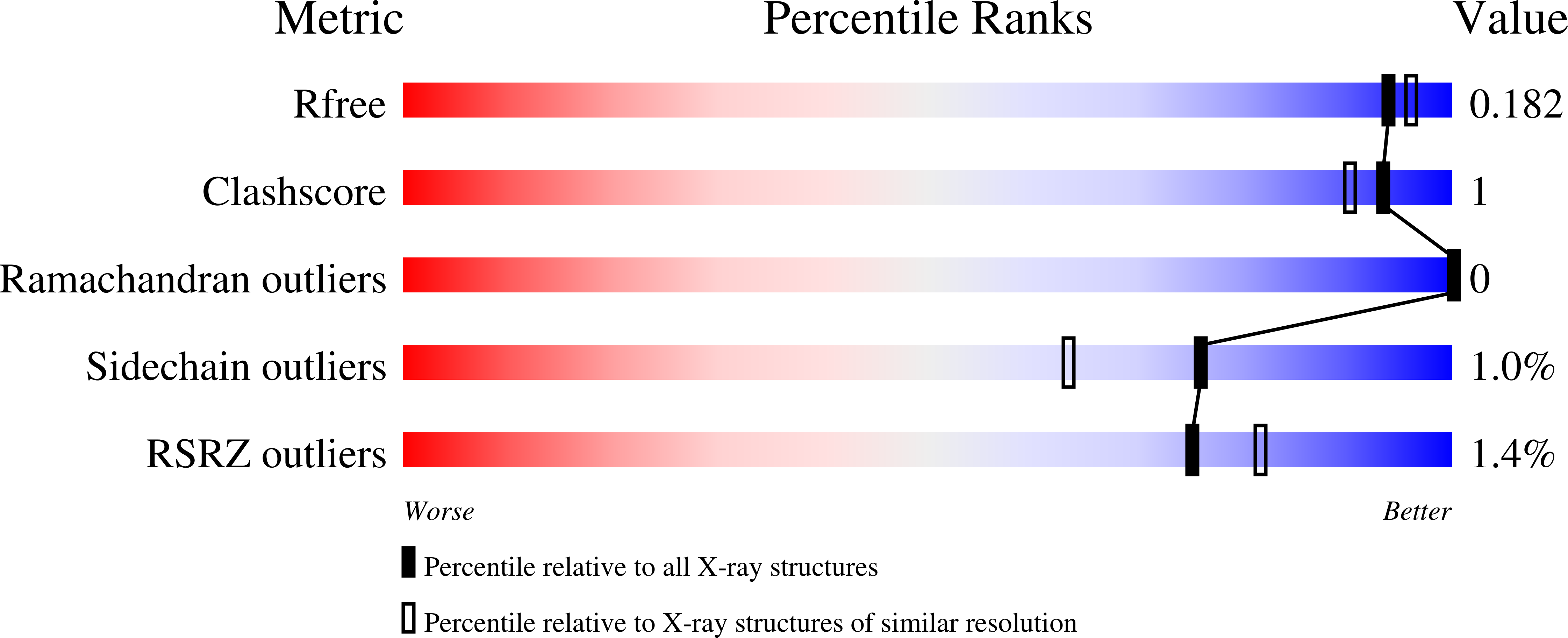
Structural and Kinetic Studies of Formate Dehydrogenase from Candida boidinii.
Guo, Q., Gakhar, L., Wickersham, K., Francis, K., Vardi-Kilshtain, A., Major, D.T., Cheatum, C.M., Kohen, A.(2016) Biochemistry 55: 2760-2771
- PubMed: 27100912
- DOI: https://doi.org/10.1021/acs.biochem.6b00181
- Primary Citation of Related Structures:
5DN9, 5DNA - PubMed Abstract:
The structure of formate dehydrogenase from Candida boidinii (CbFDH) is of both academic and practical interests. First, this enzyme represents a unique model system for studies on the role of protein dynamics in catalysis, but so far these studies have been limited by the availability of structural information. Second, CbFDH and its mutants can be used in various industrial applications (e.g., CO2 fixation or nicotinamide recycling systems), and the lack of structural information has been a limiting factor in commercial development. Here, we report the crystallization and structural determination of both holo- and apo-CbFDH. The free-energy barrier for the catalyzed reaction was computed and indicates that this structure indeed represents a catalytically competent form of the enzyme. Complementing kinetic examinations demonstrate that the recombinant CbFDH has a well-organized reactive state. Finally, a fortuitous observation has been made: the apoenzyme crystal was obtained under cocrystallization conditions with a saturating concentration of both the cofactor (NAD(+)) and inhibitor (azide), which has a nanomolar dissociation constant. It was found that the fraction of the apoenzyme present in the solution is less than 1.7 × 10(-7) (i.e., the solution is 99.9999% holoenzyme). This is an extreme case where the crystal structure represents an insignificant fraction of the enzyme in solution, and a mechanism rationalizing this phenomenon is presented.
Organizational Affiliation:
Department of Chemistry, University of Iowa , Iowa City, Iowa 52242, United States.