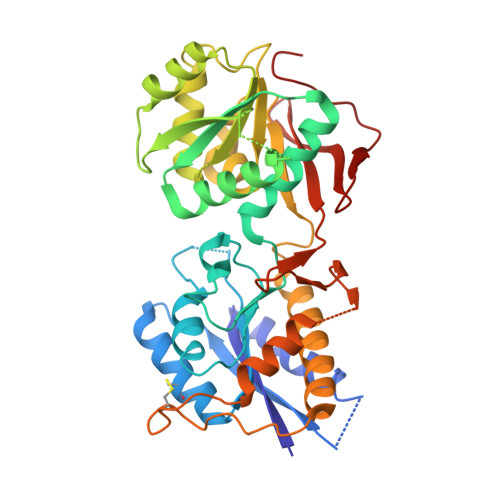
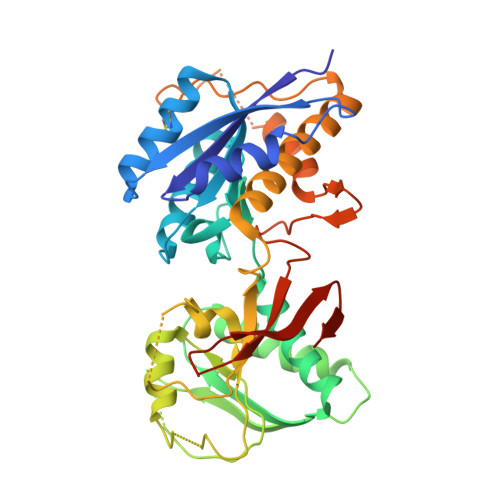
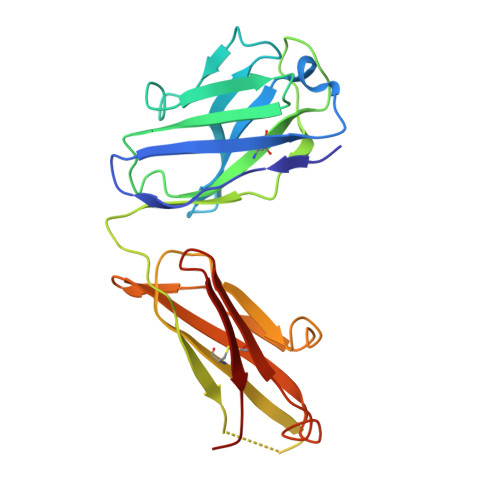
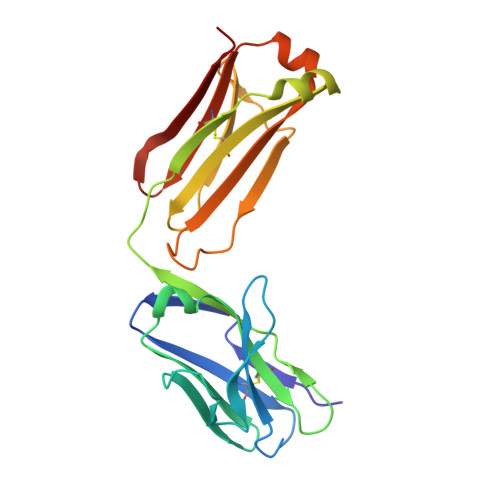
Activation of NMDA receptors and the mechanism of inhibition by ifenprodil
Tajima, N., Karakas, E., Grant, T., Simorowski, N., Diaz-Avalos, R., Grigorieff, N., Furukawa, H.(2016) Nature 534: 63-68
- PubMed: 27135925
- DOI: https://doi.org/10.1038/nature17679
- Primary Citation of Related Structures:
5B3J, 5FXG, 5FXH, 5FXI, 5FXJ, 5FXK - PubMed Abstract:
The physiology of N-methyl-d-aspartate (NMDA) receptors is fundamental to brain development and function. NMDA receptors are ionotropic glutamate receptors that function as heterotetramers composed mainly of GluN1 and GluN2 subunits. Activation of NMDA receptors requires binding of neurotransmitter agonists to a ligand-binding domain (LBD) and structural rearrangement of an amino-terminal domain (ATD). Recent crystal structures of GluN1-GluN2B NMDA receptors bound to agonists and an allosteric inhibitor, ifenprodil, represent the allosterically inhibited state. However, how the ATD and LBD move to activate the NMDA receptor ion channel remains unclear. Here we applied X-ray crystallography, single-particle electron cryomicroscopy and electrophysiology to rat NMDA receptors to show that, in the absence of ifenprodil, the bi-lobed structure of GluN2 ATD adopts an open conformation accompanied by rearrangement of the GluN1-GluN2 ATD heterodimeric interface, altering subunit orientation in the ATD and LBD and forming an active receptor conformation that gates the ion channel.
Organizational Affiliation:
Cold Spring Harbor Laboratory, W. M. Keck Structural Biology Laboratory, Cold Spring Harbor, New York 11724, USA.