Potent and Selective KDM5 Inhibitor Stops Cellular Demethylation of H3K4me3 at Transcription Start Sites and Proliferation of MM1S Myeloma Cells.
Tumber, A., Nuzzi, A., Hookway, E.S., Hatch, S.B., Velupillai, S., Johansson, C., Kawamura, A., Savitsky, P., Yapp, C., Szykowska, A., Wu, N., Bountra, C., Strain-Damerell, C., Burgess-Brown, N.A., Ruda, G.F., Fedorov, O., Munro, S., England, K.S., Nowak, R.P., Schofield, C.J., La Thangue, N.B., Pawlyn, C., Davies, F., Morgan, G., Athanasou, N., Muller, S., Oppermann, U., Brennan, P.E.(2017) Cell Chem Biol 24: 371-380
- PubMed: 28262558
- DOI: https://doi.org/10.1016/j.chembiol.2017.02.006
- Primary Citation of Related Structures:
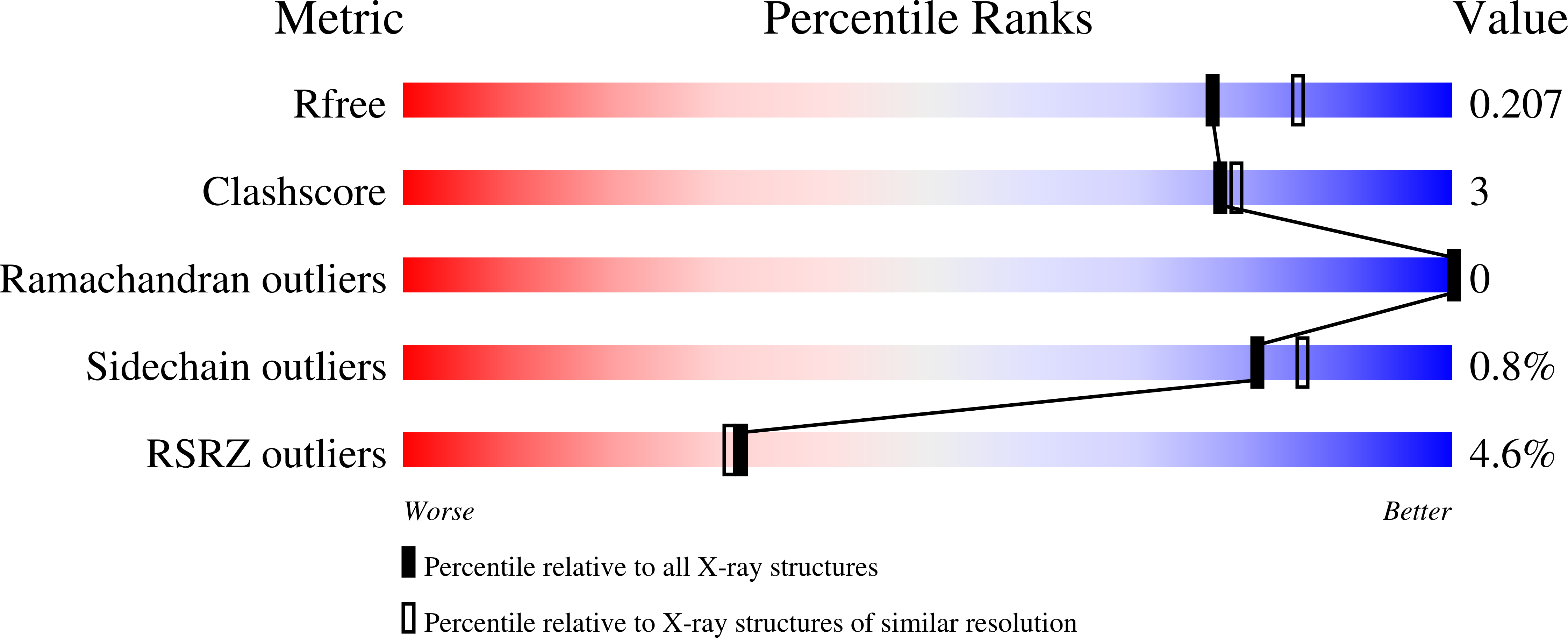
5A3N - PubMed Abstract:
Methylation of lysine residues on histone tail is a dynamic epigenetic modification that plays a key role in chromatin structure and gene regulation. Members of the KDM5 (also known as JARID1) sub-family are 2-oxoglutarate (2-OG) and Fe 2+ -dependent oxygenases acting as histone 3 lysine 4 trimethyl (H3K4me3) demethylases, regulating proliferation, stem cell self-renewal, and differentiation. Here we present the characterization of KDOAM-25, an inhibitor of KDM5 enzymes. KDOAM-25 shows biochemical half maximal inhibitory concentration values of <100 nM for KDM5A-D in vitro, high selectivity toward other 2-OG oxygenases sub-families, and no off-target activity on a panel of 55 receptors and enzymes. In human cell assay systems, KDOAM-25 has a half maximal effective concentration of ∼50 μM and good selectivity toward other demethylases. KDM5B is overexpressed in multiple myeloma and negatively correlated with the overall survival. Multiple myeloma MM1S cells treated with KDOAM-25 show increased global H3K4 methylation at transcriptional start sites and impaired proliferation.
Organizational Affiliation:
Structural Genomics Consortium, University of Oxford, Oxford OX3 7DQ, UK; Nuffield Department of Medicine, Target Discovery Institute, University of Oxford, Oxford OX3 7FZ, UK.