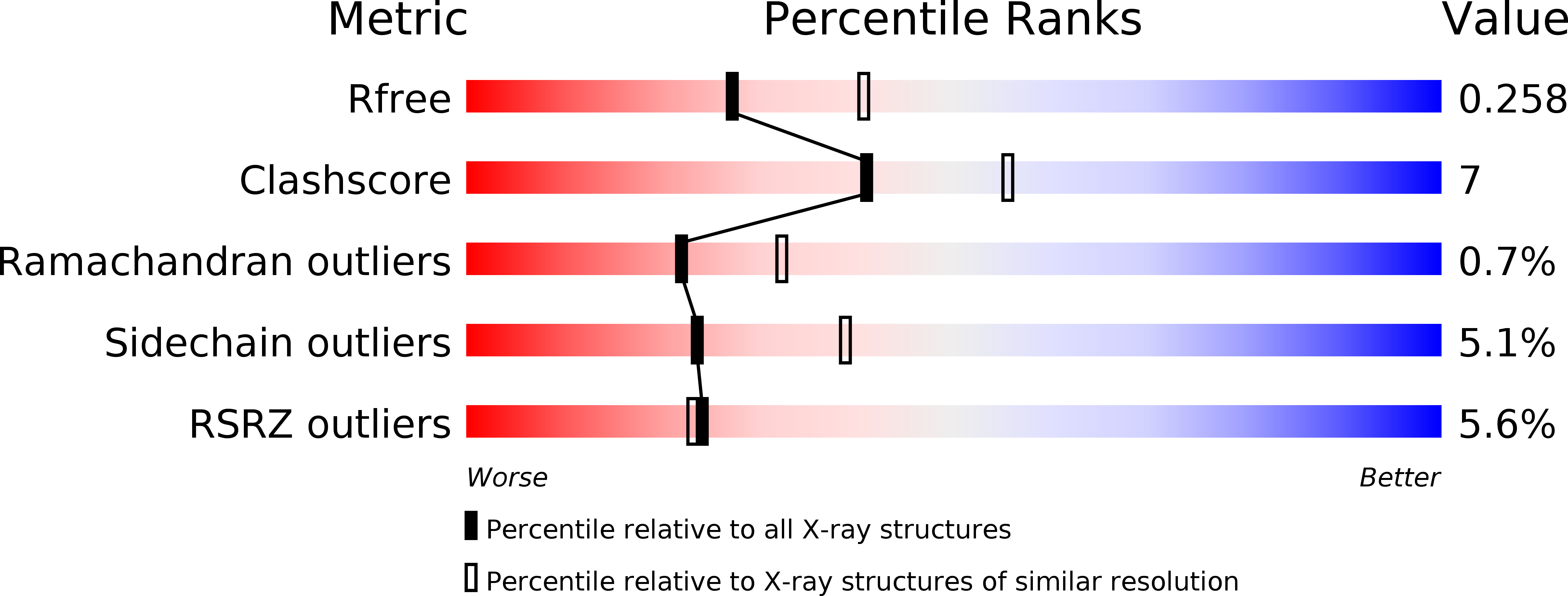
Crystal structure of the mineralocorticoid receptor DNA binding domain in complex with DNA.
Hudson, W.H., Youn, C., Ortlund, E.A.(2014) PLoS One 9: e107000-e107000
- PubMed: 25188500
- DOI: https://doi.org/10.1371/journal.pone.0107000
- Primary Citation of Related Structures:
4TNT - PubMed Abstract:
The steroid hormone receptors regulate important physiological functions such as reproduction, metabolism, immunity, and electrolyte balance. Mutations within steroid receptors result in endocrine disorders and can often drive cancer formation and progression. Despite the conserved three-dimensional structure shared among members of the steroid receptor family and their overlapping DNA binding preference, activation of individual steroid receptors drive unique effects on gene expression. Here, we present the first structure of the human mineralocorticoid receptor DNA binding domain, in complex with a canonical DNA response element. The overall structure is similar to the glucocorticoid receptor DNA binding domain, but small changes in the mode of DNA binding and lever arm conformation may begin to explain the differential effects on gene regulation by the mineralocorticoid and glucocorticoid receptors. In addition, we explore the structural effects of mineralocorticoid receptor DNA binding domain mutations found in type I pseudohypoaldosteronism and multiple types of cancer.
Organizational Affiliation:
Department of Biochemistry, Emory University School of Medicine, Atlanta, Georgia, United States of America; Discovery and Developmental Therapeutics, Winship Cancer Institute, Emory University School of Medicine, Atlanta, Georgia, United States of America.