Crystal structure of the Src tyrosine kinase SH3 domain S94A/Q128R mutant in complex with the high affinity synthetic peptide APP12
Camara-Artigas, A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Proto-oncogene tyrosine-protein kinase Src | 61 | Gallus gallus | Mutation(s): 2 Gene Names: SRC EC: 2.7.10.2 | 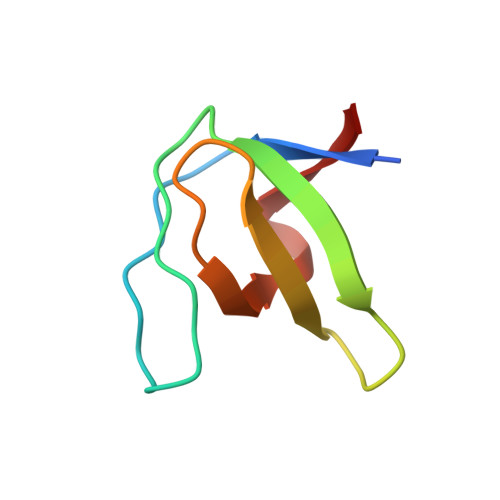 | |
UniProt | |||||
Find proteins for P00523 (Gallus gallus) Explore P00523 Go to UniProtKB: P00523 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00523 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| APP12 peptide | 13 | synthetic construct | Mutation(s): 0 | 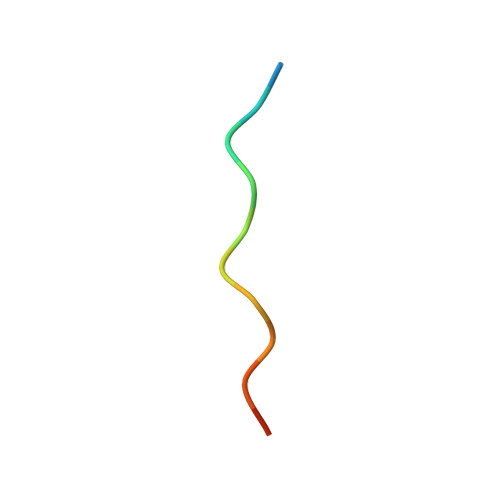 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 31.559 | α = 90 |
| b = 31.559 | β = 90 |
| c = 106.715 | γ = 120 |
| Software Name | Purpose |
|---|---|
| Aimless | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| MOSFLM | data reduction |
| autoPROC | data scaling |