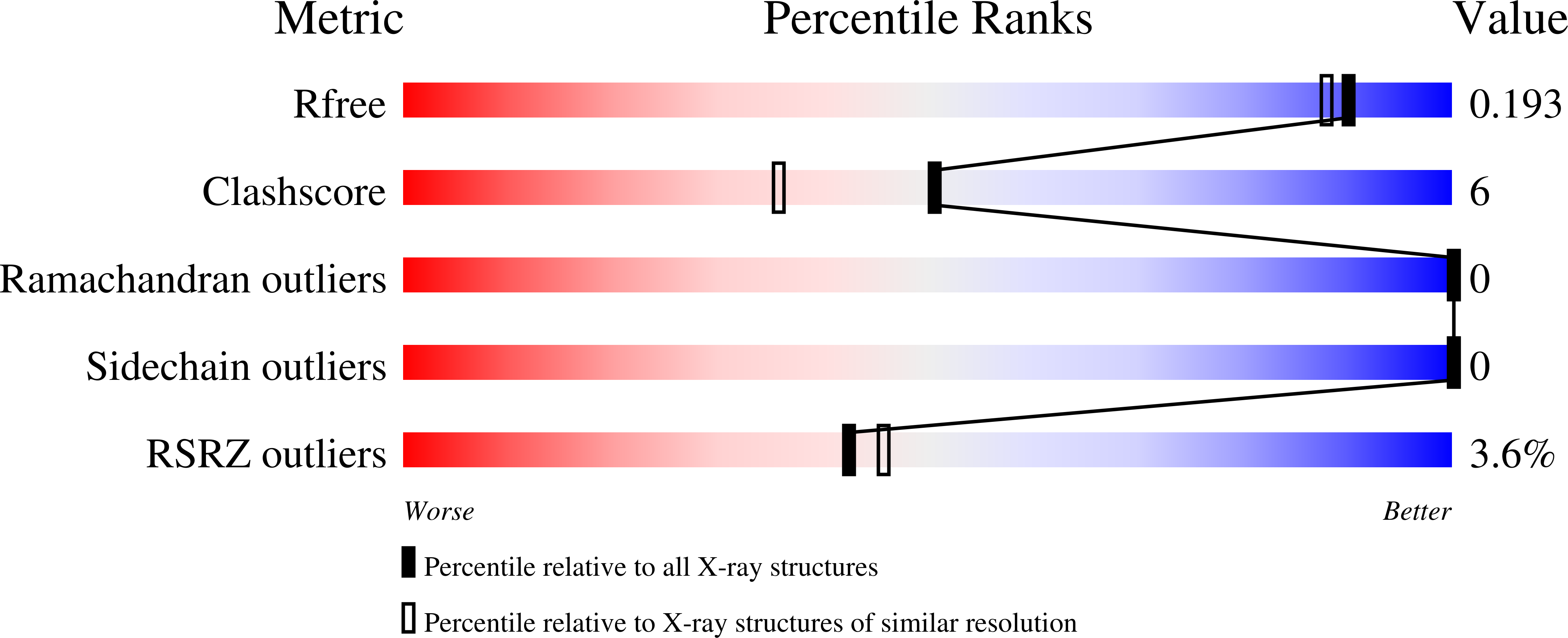
Conformationally constrained nucleoside phosphonic acids - potent inhibitors of human mitochondrial and cytosolic 5'(3')-nucleotidases.
Simak, O., Pachl, P., Fabry, M., Budesinsky, M., Jandusik, T., Hnizda, A., Sklenickova, R., Petrova, M., Veverka, V., Rezacova, P., Brynda, J., Rosenberg, I.(2014) Org Biomol Chem 12: 7971-7982
- PubMed: 25178098
- DOI: https://doi.org/10.1039/c4ob01332h
- Primary Citation of Related Structures:
4L6C, 4MWO, 4NFL - PubMed Abstract:
This work describes novel in vitro inhibitors of human mitochondrial (mdN) and cytosolic (cdN) 5'(3')-deoxynucleotidases. We designed a series of derivatives of the lead compound (S)-1-[2-deoxy-3,5-O-(phosphonobenzylidene)-β-d-threo-pentofuranosyl]thymine bearing various substituents in the para position of the benzylidene moiety. Detailed kinetic study revealed that certain para substituents increase the inhibitory potency (iodo derivative; K = 2.71 μM) and some induce a shift in selectivity toward cdN (carboxy derivative, K = 11.60 μM; iodoxy derivative, K = 6.60 μM). Crystal structures of mdN in complex with three of these compounds revealed that various para substituents lead to two alternative inhibitor binding modes within the enzyme active site. Two binding modes were also identified for cdN complexes by heteronuclear NMR spectroscopy.
Organizational Affiliation:
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, v. v. i., Flemingovo 2, 166 10 Prague 6, Czech Republic. rosenberg@uochb.cas.cz brynda@img.cas.cz.