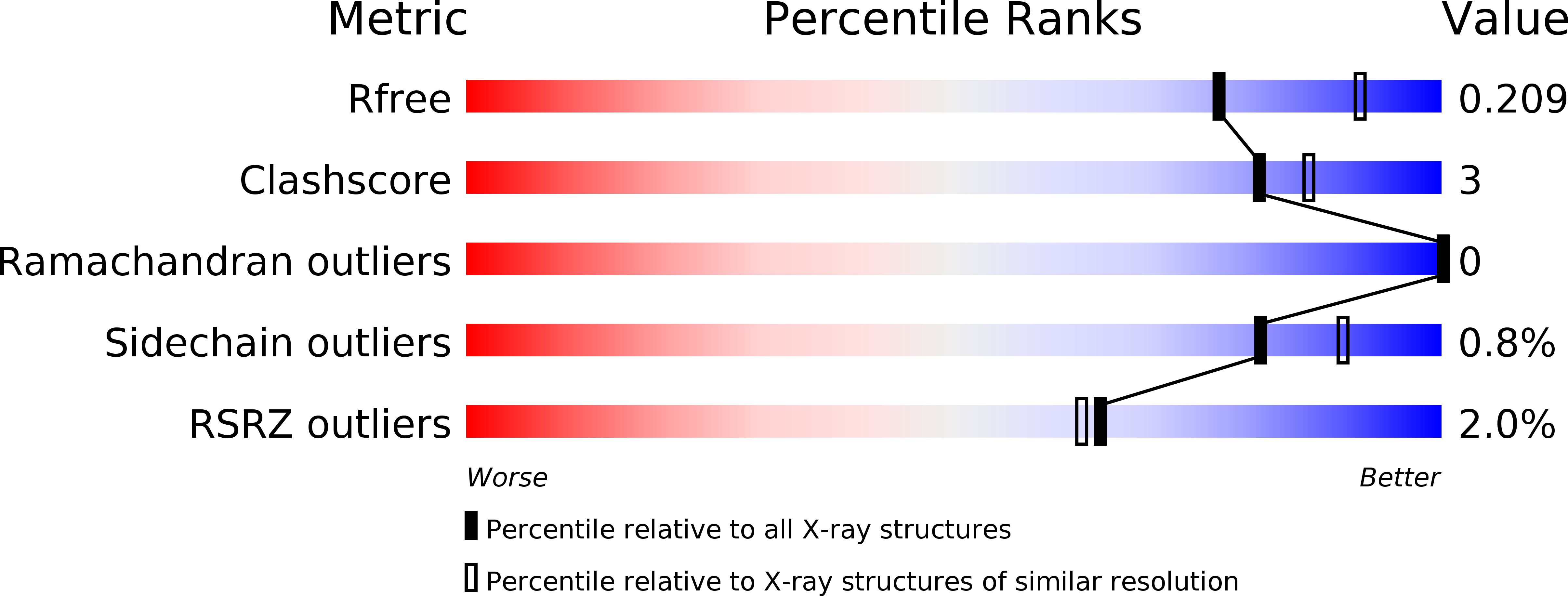
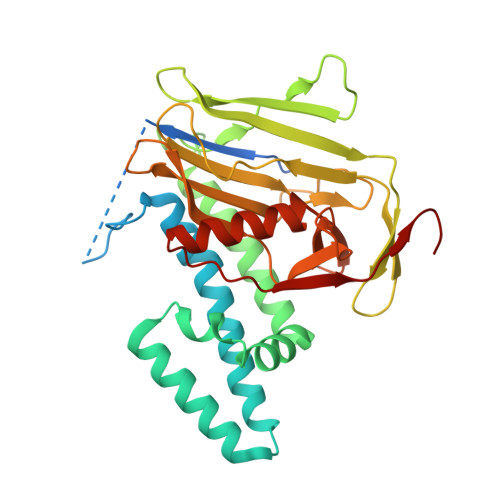
Crystal structure of the N-terminal domains of the surface cell antigen 4 of Rickettsia.
Lee, J.H., Vonrhein, C., Bricogne, G., Izard, T.(2013) Protein Sci 22: 1425-1431
- PubMed: 23904352
- DOI: https://doi.org/10.1002/pro.2322
- Primary Citation of Related Structures:
4LQ8 - PubMed Abstract:
The obligate intracellular, gram-negative bacterium Rickettsia is the causative agent of spotted fevers and typhus in humans. Surface cell antigen (sca) proteins surround these bacteria. We recently reported the co-localization of one of these proteins, sca4, with vinculin in cells at sites of focal adhesions and demonstrated that two vinculin binding sites directed the sca4/vinculin interaction. Here we report the 2.2 Å crystal structure of the conserved N-terminal 38 kDa domain of sca4 from Rickettsia rickettsii. The structure reveals two subdomains. The first is an all-helical domain that is folded in a fashion similar to the dimeric assembly chaperone for rubisco, namely RbcX. The following and highly conserved β-strand domain lacks significant structural similarity with other known structures and to the best of our knowledge represents a new protein fold.
Organizational Affiliation:
Department of Cancer Biology, Cell Adhesion Laboratory, The Scripps Research Institute, Jupiter, Florida, 33458.