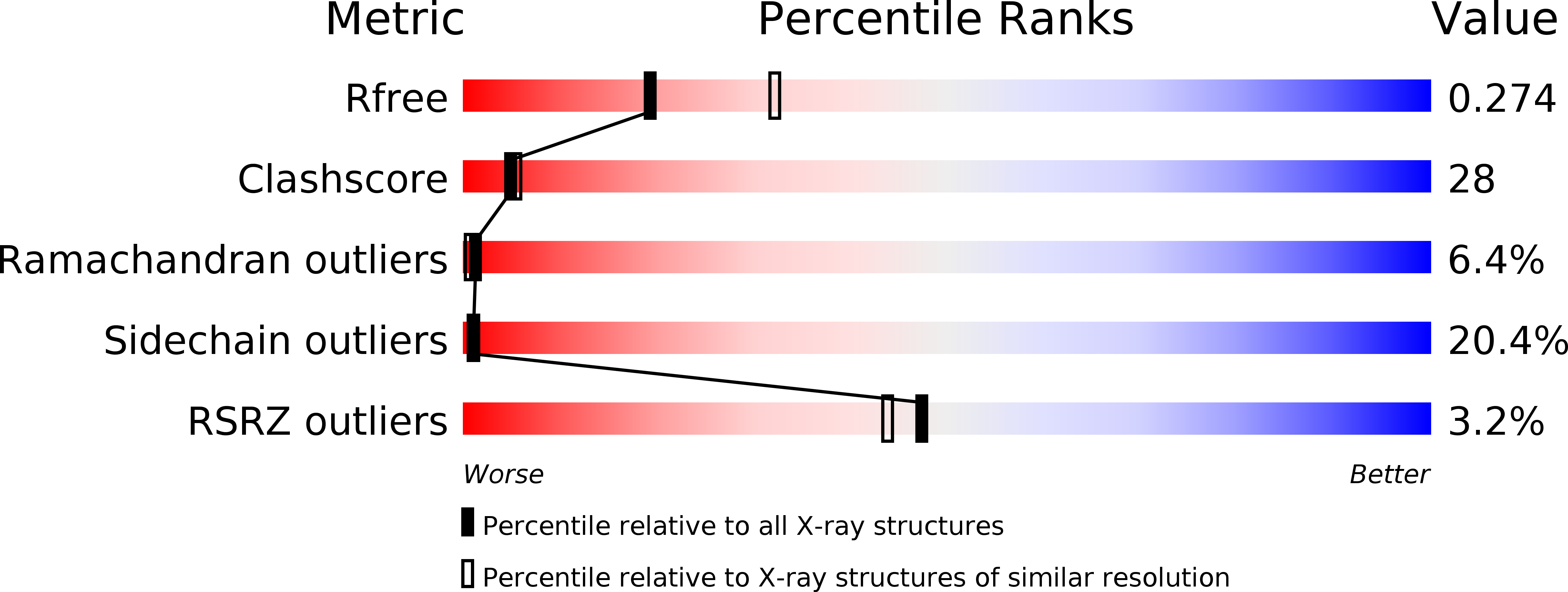
Structural Insights into the Abscisic Acid Stereospecificity by the ABA Receptors PYR/PYL/RCAR
Zhang, X., Jiang, L., Wang, G., Yu, L., Zhang, Q., Xin, Q., Wu, W., Gong, Z., Chen, Z.(2013) PLoS One 8: e67477-e67477
- PubMed: 23844015
- DOI: https://doi.org/10.1371/journal.pone.0067477
- Primary Citation of Related Structures:
3OQU, 4JDA, 4JDL - PubMed Abstract:
The phytohormone abscisic acid ((+)-ABA) plays a key role in many processes. The biological and biochemical activities of unnatural (-)-ABA have been extensively investigated since 1960s. However, the recognition mechanism by which only a few members among PYR/PYL/RCAR (PYLs) family can bind (-)-ABA remains largely unknown. Here we systematically characterized the affinity of PYLs binding to the (-)-ABA and reported the crystal structures of apo-PYL5, PYL3-(-)-ABA and PYL9-(+)-ABA. PYL5 showed the strongest binding affinity with (-)-ABA among all the PYLs. PYL9 is a stringently exclusive (+)-ABA receptor with interchangeable disulfide bonds shared by a subclass of PYLs. PYL3 is a dual receptor to both ABA enantiomers. The binding orientation and pocket of (-)-ABA in PYLs are obviously different from those of (+)-ABA. Steric hindrance and hydrophobic interaction are the two key factors in determining the stereospecificity of PYLs binding to (-)-ABA, which is further confirmed by gain-of-function and loss-of-function mutagenesis. Our results provide novel insights of the bioactivity of ABA enantiomers onto PYLs, and shed light on designing the selective ABA receptors agonists.
Organizational Affiliation:
State Key Laboratory of Agrobiotechnology, China Agricultural University, Beijing, China.