Quinone-dependent proton transfer pathways in the photosynthetic cytochrome b6f complex
Hasan, S.S., Yamashita, E., Baniulis, D., Cramer, W.A.(2013) Proc Natl Acad Sci U S A 110: 4297-4302
- PubMed: 23440205
- DOI: https://doi.org/10.1073/pnas.1222248110
- Primary Citation of Related Structures:
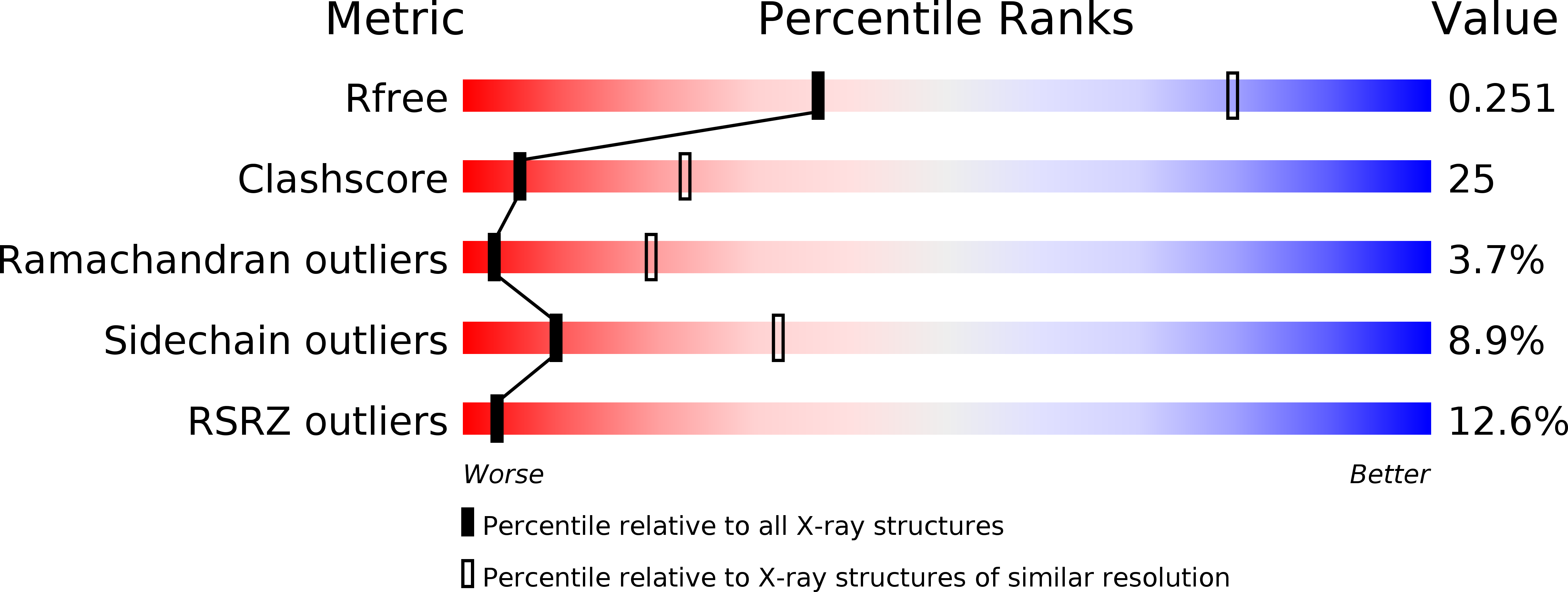
4H0L, 4H13, 4H44 - PubMed Abstract:
As much as two-thirds of the proton gradient used for transmembrane free energy storage in oxygenic photosynthesis is generated by the cytochrome b6f complex. The proton uptake pathway from the electrochemically negative (n) aqueous phase to the n-side quinone binding site of the complex, and a probable route for proton exit to the positive phase resulting from quinol oxidation, are defined in a 2.70-Å crystal structure and in structures with quinone analog inhibitors at 3.07 Å (tridecyl-stigmatellin) and 3.25-Å (2-nonyl-4-hydroxyquinoline N-oxide) resolution. The simplest n-side proton pathway extends from the aqueous phase via Asp20 and Arg207 (cytochrome b6 subunit) to quinone bound axially to heme c(n). On the positive side, the heme-proximal Glu78 (subunit IV), which accepts protons from plastosemiquinone, defines a route for H(+) transfer to the aqueous phase. These pathways provide a structure-based description of the quinone-mediated proton transfer responsible for generation of the transmembrane electrochemical potential gradient in oxygenic photosynthesis.
Organizational Affiliation:
Department of Biological Sciences, Purdue University, West Lafayette, IN 47907, USA.