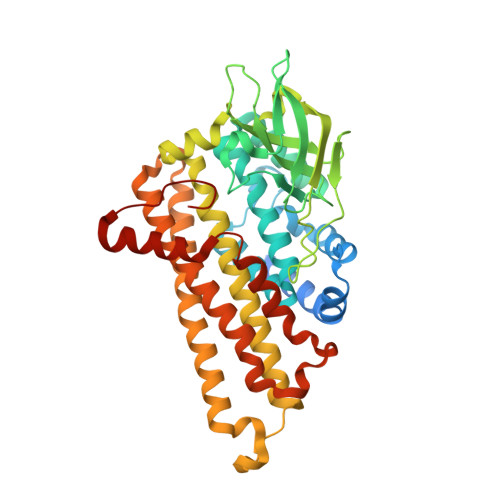
Crystal structure of DszC from Rhodococcus sp. XP at 1.79 angstrom
Liu, S., Zhang, C., Su, T., Wei, T., Zhu, D., Wang, K., Huang, Y., Dong, Y., Yin, K., Xu, S., Xu, P., Gu, L.(2014) Proteins 82: 1708-1720
- PubMed: 24470304
- DOI: https://doi.org/10.1002/prot.24525
- Primary Citation of Related Structures:
4DOY - PubMed Abstract:
The dibenzothiophene (DBT) monooxygenase DszC, which is the key initiating enzyme in "4S" metabolic pathway, catalyzes sequential sulphoxidation reaction of DBT to DBT sulfoxide (DBTO), then DBT sulfone (DBTO2). Here, we report the crystal structure of DszC from Rhodococcus sp. XP at 1.79 Å. Intriguingly, two distinct conformations occur in the flexible lid loops adjacent to the active site (residue 280-295, between α9 and α10). They are named "open"' and "closed" state respectively, and might show the status of the free and ligand-bound DszC. The molecular docking results suggest that the reduced FMN reacts with an oxygen molecule at C4a position of the isoalloxazine ring, producing the C4a-(hydro)peroxyflavin intermediate which is stabilized by H391 and S163. H391 may contribute to the formation of the C4a-(hydro)peroxyflavin by acting as a proton donor to the proximal peroxy oxygen, and it might also be involved in the protonation process of the C4a-(hydro)xyflavin. Site-directed mutagenesis study shows that mutations in the residues involved either in catalysis or in flavin or substrate-binding result in a complete loss of enzyme activity, suggesting that the accurate positions of flavin and substrate are crucial for the enzyme activity.
Organizational Affiliation:
State Key Laboratory of Microbial Technology, School of Life Sciences, Shandong University, Jinan, 250100, China.