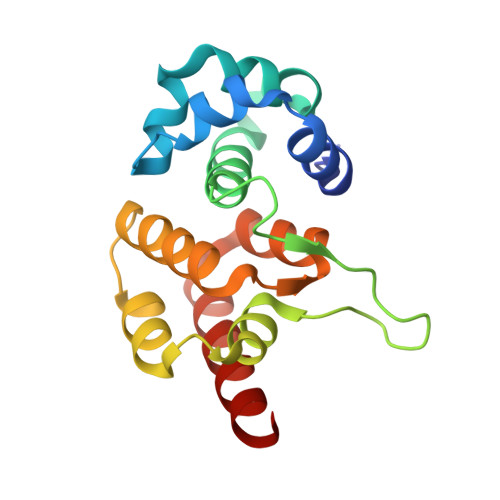
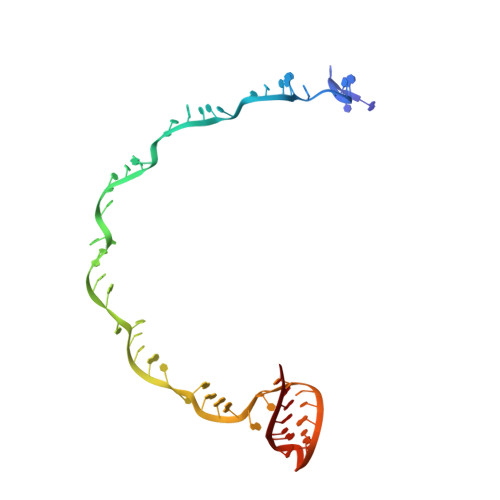
Crystal structure of the RNA-guided immune surveillance Cascade complex in Escherichia coli
Zhao, H., Sheng, G., Wang, J., Wang, M., Bunkoczi, G., Gong, W., Wei, Z., Wang, Y.(2014) Nature 515: 147-150
- PubMed: 25118175
- DOI: https://doi.org/10.1038/nature13733
- Primary Citation of Related Structures:
4U7U - PubMed Abstract:
Clustered regularly interspaced short palindromic repeats (CRISPR) together with CRISPR-associated (Cas) proteins form the CRISPR/Cas system to defend against foreign nucleic acids of bacterial and archaeal origin. In the I-E subtype CRISPR/Cas system, eleven subunits from five Cas proteins (CasA1B2C6D1E1) assemble along a CRISPR RNA (crRNA) to form the Cascade complex. Here we report on the 3.05 Å crystal structure of the 405-kilodalton Escherichia coli Cascade complex that provides molecular details beyond those available from earlier lower-resolution cryo-electron microscopy structures. The bound 61-nucleotide crRNA spans the entire 11-protein subunit-containing complex, where it interacts with all six CasC subunits (named CasC1-6), with its 5' and 3' terminal repeats anchored by CasD and CasE, respectively. The crRNA spacer region is positioned along a continuous groove on the concave surface generated by the aligned CasC1-6 subunits. The five long β-hairpins that project from individual CasC2-6 subunits extend across the crRNA, with each β-hairpin inserting into the gap between the last stacked base and its adjacent splayed counterpart, and positioned within the groove of the preceding CasC subunit. Therefore, instead of continuously stacking, the crRNA spacer region is divided into five equal fragments, with each fragment containing five stacked bases flanked by one flipped-out base. Each of those crRNA spacer fragments interacts with CasC in a similar fashion. Furthermore, our structure explains why the seed sequence, with its outward-directed bases, has a critical role in target DNA recognition. In conclusion, our structure of the Cascade complex provides novel molecular details of protein-protein and protein-RNA alignments and interactions required for generation of a complex mediating RNA-guided immune surveillance.
Organizational Affiliation:
1] Laboratory of RNA Biology, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China [2] University of Chinese Academy of Sciences, Beijing 100049, China.