Selectivity of Ni(II) and Zn(II) binding to Sporosarcina pasteurii UreE, a metallochaperone in the urease assembly: a calorimetric and crystallographic study.
Zambelli, B., Banaszak, K., Merloni, A., Kiliszek, A., Rypniewski, W., Ciurli, S.(2013) J Biol Inorg Chem 18: 1005-1017
- PubMed: 24126709
- DOI: https://doi.org/10.1007/s00775-013-1049-6
- Primary Citation of Related Structures:
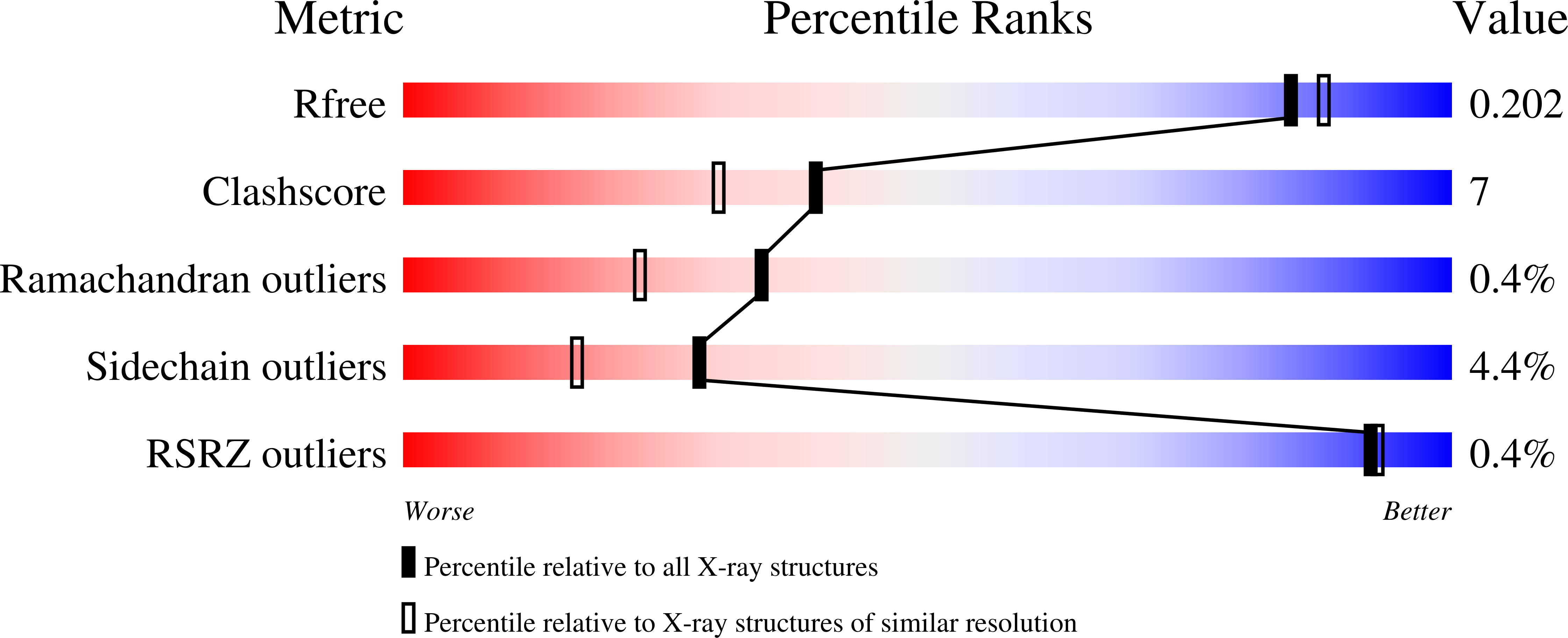
4L3K - PubMed Abstract:
Urease is a nickel-dependent enzyme that plays a critical role in the biogeochemical nitrogen cycle by catalyzing the hydrolysis of urea to ammonia and carbamate. This enzyme, initially synthesized in the apo form, needs to be activated by incorporation of two nickel ions into the active site, a process driven by the dimeric metallochaperone UreE. Previous studies reported that this protein can bind different metal ions in vitro, beside the cognate Ni(II). This study explores the metal selectivity and affinity of UreE from Sporosarcina pasteurii (Sp, formerly known as Bacillus pasteurii) for cognate [Ni(II)] and noncognate [Zn(II)] metal ions. In particular, the thermodynamic parameters of SpUreE Ni(II) and Zn(II) binding have been determined using isothermal titration calorimetry. These experiments show that two Ni(II) ions bind to the protein dimer with positive cooperativity. The high-affinity site involves the conserved solvent-exposed His(100) and the C-terminal His(145), whereas the low-affinity site comprises also the C-terminal His(147). Zn(II) binding to the protein, occurring in the same protein regions and with similar affinity as compared to Ni(II), causes metal-driven dimerization of the protein dimer. The crystal structure of the protein obtained in the presence of equimolar amounts of both metal ions indicates that the high-affinity metal binding site binds Ni(II) preferentially over Zn(II). The ability of the protein to select Ni(II) over Zn(II) was confirmed by competition experiments in solution as well as by analysis of X-ray anomalous dispersion data. Overall, the thermodynamics and structural parameters that modulate the metal ion specificity of the different binding sites on the protein surface of SpUreE have been established.
Organizational Affiliation:
Laboratory of Bioinorganic Chemistry, Department of Pharmacy and Biotechnology, University of Bologna, Bologna, Italy.