Functional and structural characterization of the mammalian TREX-2 complex that links transcription with nuclear messenger RNA export.
Jani, D., Lutz, S., Hurt, E., Laskey, R.A., Stewart, M., Wickramasinghe, V.O.(2012) Nucleic Acids Res 40: 4562-4573
- PubMed: 22307388
- DOI: https://doi.org/10.1093/nar/gks059
- Primary Citation of Related Structures:
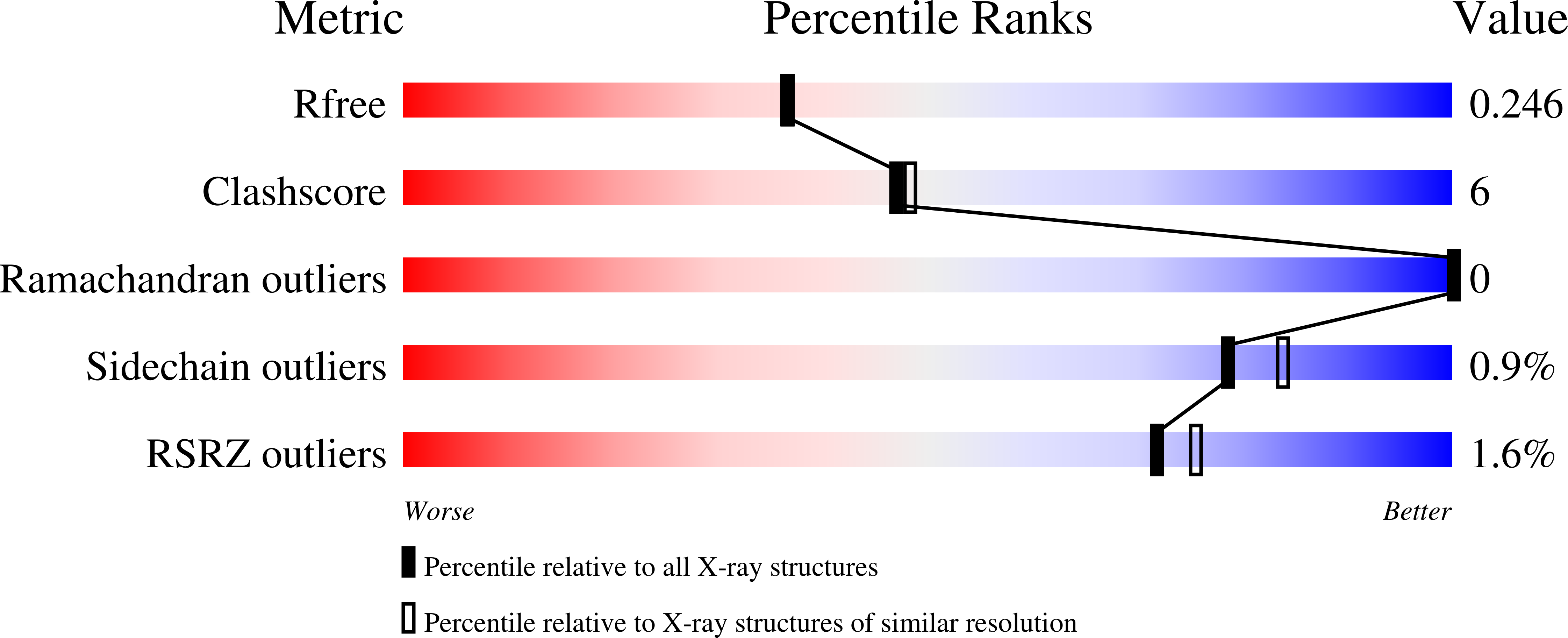
4DHX - PubMed Abstract:
Export of messenger RNA (mRNA) from the nucleus to the cytoplasm is a critical step in the gene expression pathway of eukaryotic cells. Here, we report the functional and structural characterization of the mammalian TREX-2 complex and show how it links transcription/processing with nuclear mRNA export. Mammalian TREX-2 is based on a germinal-centre associated nuclear protein (GANP) scaffold to which ENY2, PCID2 and centrins bind and depletion of any of these components inhibits mRNA export. The crystal structure of the GANP:ENY2 complex shows that two ENY2 chains interact directly with GANP, but they have different orientations from those observed on yeast Sac3. GANP is required to recruit ENY2 to nuclear pore complexes (NPCs), but ENY2 is not necessary to recruit GANP, which requires both its CID and MCM3AP domains, together with nucleoporin Nup153. GANP and ENY2 associate with RNA polymerase II and inhibition of mRNA processing redistributes GANP from NPCs into nuclear foci indicating that mammalian TREX-2 is associated with transcription. Thus, we implicate TREX-2 as an integral component of the mammalian mRNA export machinery where it links transcription and nuclear export by facilitating the transfer of mature mRNPs from the nuclear interior to NPCs.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Hills Road, Cambridge CB2 0QH, UK.