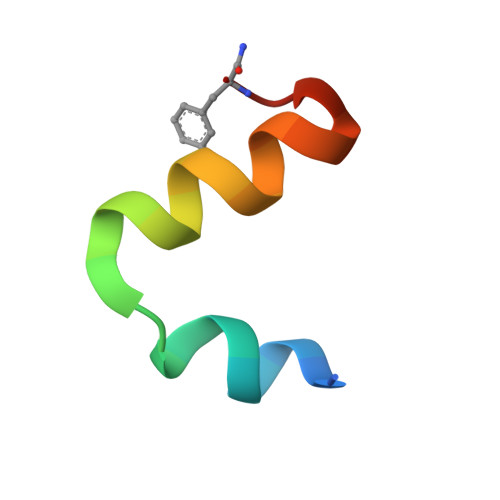
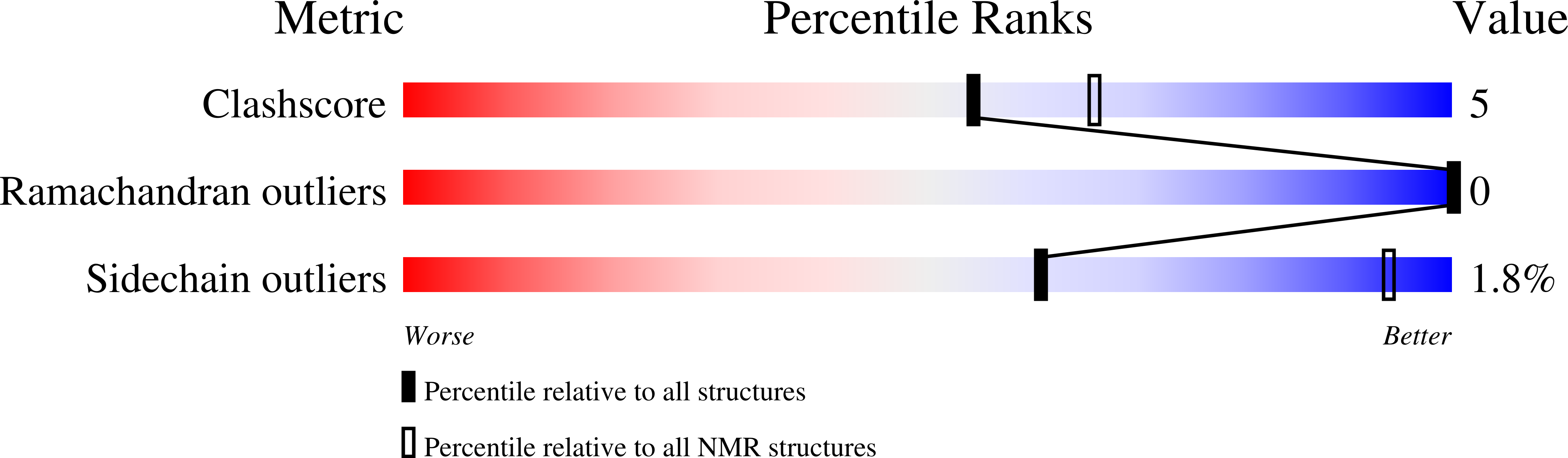A Compact Native 24-Residue Supersecondary Structure Derived from the Villin Headpiece Subdomain.
Hocking, H.G., Hase, F., Madl, T., Zacharias, M., Rief, M., Zoldak, G.(2015) Biophys J 108: 678
- PubMed: 25650934
- DOI: https://doi.org/10.1016/j.bpj.2014.11.3482
- Primary Citation of Related Structures:
4CZ3, 4CZ4 - PubMed Abstract:
Many small proteins fold highly cooperatively in an all-or-none fashion and thus their native states are well protected from thermal fluctuations by an extensive network of interactions across the folded structure. Because protein structures are stabilized by local and nonlocal interactions among distal residues, dissecting individual substructures from the context of folded proteins results in large destabilization and loss of unique three-dimensional structure. Thus, mini-protein substructures can only rarely be derived from natural templates. Here, we describe a compact native 24-residues-long supersecondary structure derived from the hyperstable villin headpiece subdomain consisting of helices 2 and 3 (HP24). Using a combination of experimental techniques, including NMR and small-angle x-ray scattering, as well as all-atom replica exchange molecular-dynamics simulations, we show that a variant with stabilizing substitutions (HP24stab) forms a densely packed and compact conformation. In HP24stab, interactions between helices 2 and 3 are similar to those observed in native folded HP35, and the two helices cooperatively stabilize each other by completing the hydrophobic core lining the central part of HP35. Interestingly, even though the HP24wt fragment shows a more expanded and less structured conformation, NMR and simulations demonstrate a preference for a native-like topology. Thus, the two stabilizing residues in HP24stab shift the energy balance toward the native state, leading to a minimal folding motif.
Organizational Affiliation:
Center for Integrated Protein Science Munich, Department Chemie, Technische Universität München, Garching, Germany; Institute of Structural Biology, Helmholtz Zentrum München, Neuherberg, Germany.