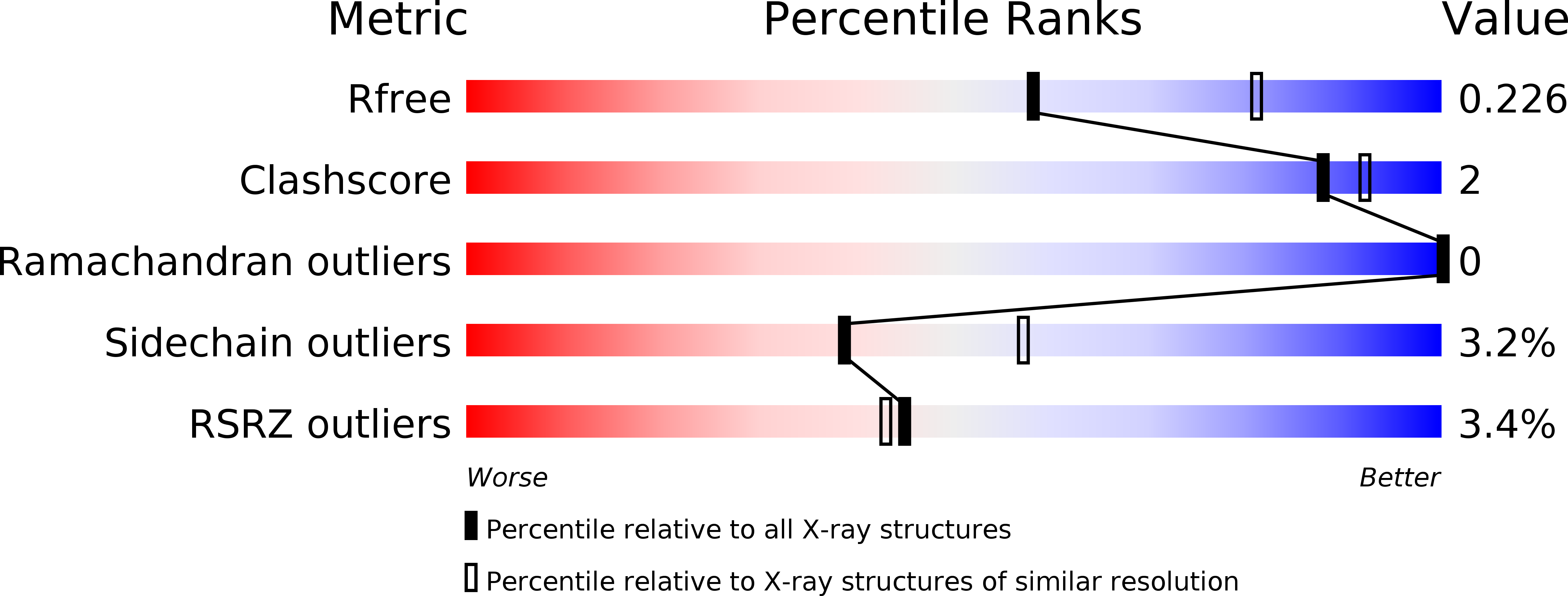
Synthetic symmetrization in the crystallization and structure determination of CelA from Thermotoga maritima.
Forse, G.J., Ram, N., Banatao, D.R., Cascio, D., Sawaya, M.R., Klock, H.E., Lesley, S.A., Yeates, T.O.(2011) Protein Sci 20: 168-178
- PubMed: 21082721
- DOI: https://doi.org/10.1002/pro.550
- Primary Citation of Related Structures:
3O7O - PubMed Abstract:
Protein crystallization continues to be a major bottleneck in X-ray crystallography. Previous studies suggest that symmetric proteins, such as homodimers, might crystallize more readily than monomeric proteins or asymmetric complexes. Proteins that are naturally monomeric can be made homodimeric artificially. Our approach is to create homodimeric proteins by introducing single cysteines into the protein of interest, which are then oxidized to form a disulfide bond between the two monomers. By introducing the single cysteine at different sequence positions, one can produce a variety of synthetically dimerized versions of a protein, with each construct expected to exhibit its own crystallization behavior. In earlier work, we demonstrated the potential utility of the approach using T4 lysozyme as a model system. Here we report the successful application of the method to Thermotoga maritima CelA, a thermophilic endoglucanase enzyme with low sequence identity to proteins with structures previously reported in the Protein Data Bank. This protein had resisted crystallization in its natural monomeric form, despite a broad survey of crystallization conditions. The synthetic dimerization of the CelA mutant D188C yielded well-diffracting crystals with molecules in a packing arrangement that would not have occurred with native, monomeric CelA. A 2.4 Å crystal structure was determined by single anomalous dispersion using a seleno-methionine derivatized protein. The results support the notion that synthetic symmetrization can be a useful approach for enlarging the search space for crystallizing monomeric proteins or asymmetric complexes.
Organizational Affiliation:
Department of Chemistry and Biochemistry, UCLA, Los Angeles, California 90095, USA.