Structure and mechanism of receptor sharing by the IL-10R2 common chain.
Yoon, S.I., Jones, B.C., Logsdon, N.J., Harris, B.D., Deshpande, A., Radaeva, S., Halloran, B.A., Gao, B., Walter, M.R.(2010) Structure 18: 638-648
- PubMed: 20462497
- DOI: https://doi.org/10.1016/j.str.2010.02.009
- Primary Citation of Related Structures:
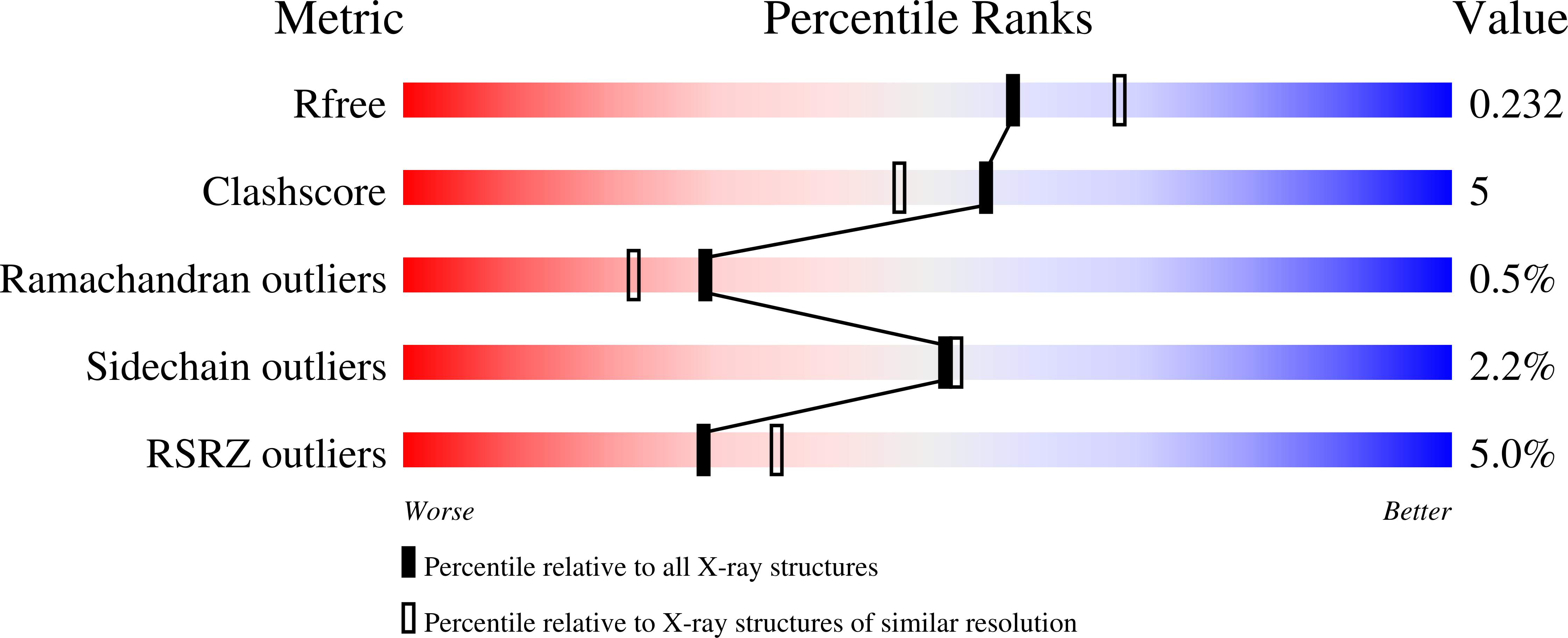
3LQM - PubMed Abstract:
IL-10R2 is a shared cell surface receptor required for the activation of five class 2 cytokines (IL-10, IL-22, IL-26, IL-28, and IL-29) that play critical roles in host defense. To define the molecular mechanisms that regulate its promiscuous binding, we have determined the crystal structure of the IL-10R2 ectodomain at 2.14 A resolution. IL-10R2 residues required for binding were identified by alanine scanning and used to derive computational models of IL-10/IL-10R1/IL-10R2 and IL-22/IL-22R1/IL-10R2 ternary complexes. The models reveal a conserved binding epitope that is surrounded by two clefts that accommodate the structural and chemical diversity of the cytokines. These results provide a structural framework for interpreting IL-10R2 single nucleotide polymorphisms associated with human disease.
Organizational Affiliation:
Department of Microbiology, University of Alabama at Birmingham, Birmingham, AL 35294, USA.