Crystal structure and functional insights of hemopexin fold protein from grass pea
Gaur, V., Qureshi, I.A., Singh, A., Chanana, V., Salunke, D.M.(2010) Plant Physiol 152: 1842-1850
- PubMed: 20147493
- DOI: https://doi.org/10.1104/pp.109.150680
- Primary Citation of Related Structures:
3LP9 - PubMed Abstract:
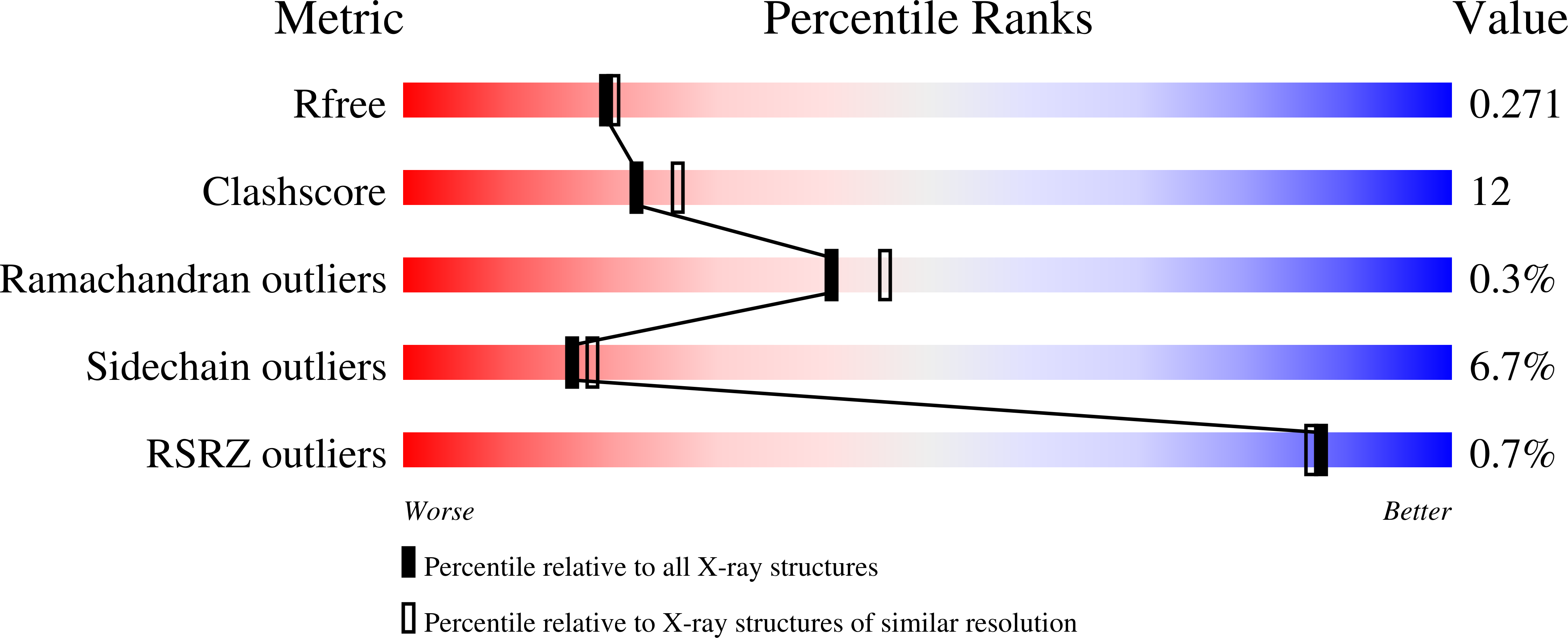
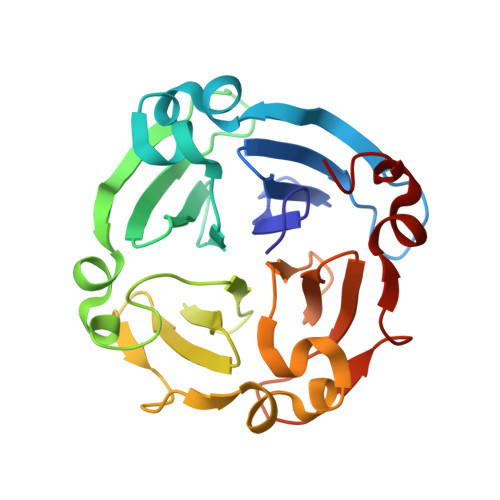
A regulatory protein from grass pea (Lathyrus sativus), LS-24, a close homolog of albumin 2 from garden pea (Pisum sativum) that is associated with polyamine biosynthesis, was characterized and the structure of a hemopexin-type fold among plant proteins illustrated. Crystal structure of LS-24 determined at 2.2 A resolution by multiple isomorphous replacement phasing showed four-bladed beta-propeller structure having a pseudo 4-fold molecular symmetry along a metal ion-binding central channel. The structure represents typical mammalian hemopexin fold with discernible features correlated with the possible functional variations. The protein was found to exist in the dimeric state. While LS-24 dimer binds to spermine in the crystal structure as well as in solution, binding of heme in solution resulted in the dissociation of the dimer into monomers with concomitant release of bound spermine. Interactions of heme and spermine with LS-24 bear physiological implications. While binding of spermine to LS-24 can be linked with polyamine biosynthesis that of heme correlates with oxidative stress. Mutually exclusive binding of heme and spermine in different oligomeric states suggest a role for LS-24 in sensing oxidative stress through a ligand-regulated monomer-dimer transition switch.
Organizational Affiliation:
National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi 110 067, India.