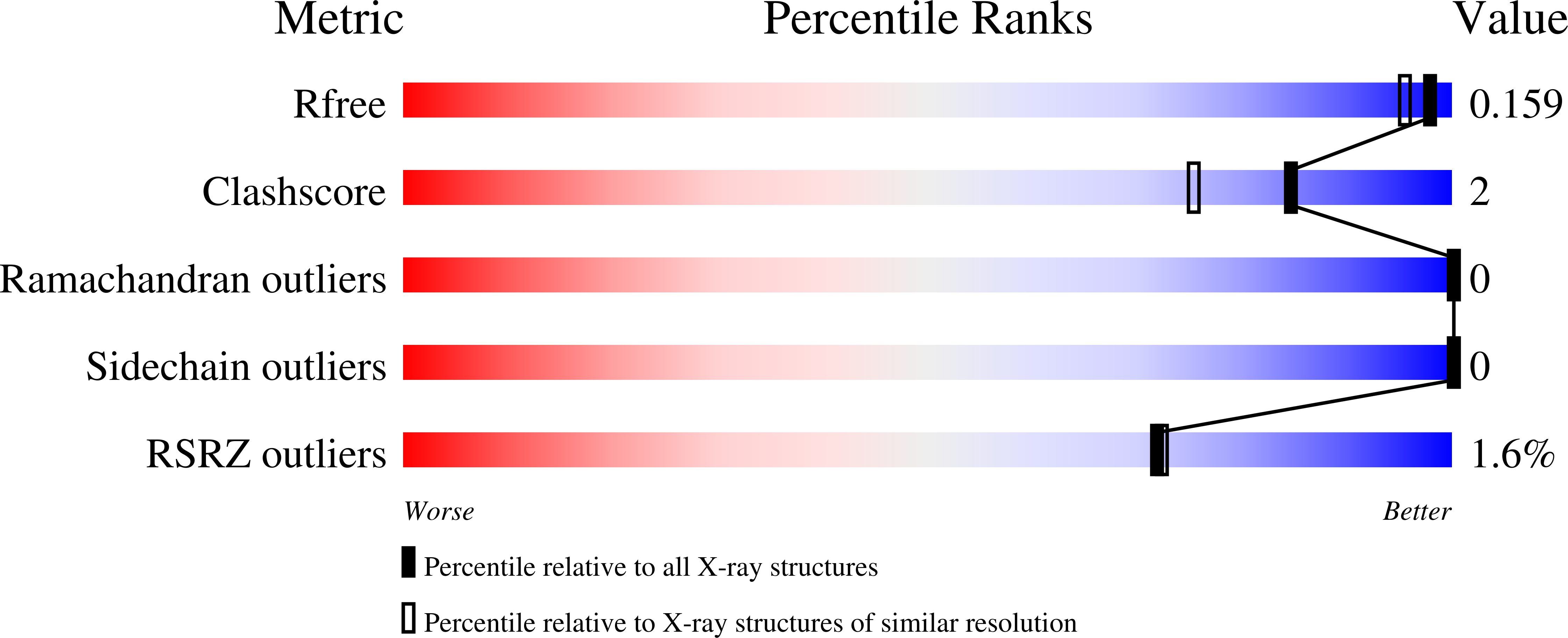
The structure of Jann_2411 (DUF1470) from Jannaschia sp. at 1.45 resolution reveals a new fold (the ABATE domain) and suggests its possible role as a transcription regulator.
Bakolitsa, C., Bateman, A., Jin, K.K., McMullan, D., Krishna, S.S., Miller, M.D., Abdubek, P., Acosta, C., Astakhova, T., Axelrod, H.L., Burra, P., Carlton, D., Chiu, H.J., Clayton, T., Das, D., Deller, M.C., Duan, L., Elias, Y., Feuerhelm, J., Grant, J.C., Grzechnik, A., Grzechnik, S.K., Han, G.W., Jaroszewski, L., Klock, H.E., Knuth, M.W., Kozbial, P., Kumar, A., Marciano, D., Morse, A.T., Murphy, K.D., Nigoghossian, E., Okach, L., Oommachen, S., Paulsen, J., Reyes, R., Rife, C.L., Sefcovic, N., Tien, H., Trame, C.B., Trout, C.V., van den Bedem, H., Weekes, D., White, A., Xu, Q., Hodgson, K.O., Wooley, J., Elsliger, M.A., Deacon, A.M., Godzik, A., Lesley, S., Wilson, I.A.(2010) Acta Crystallogr Sect F Struct Biol Cryst Commun 66: 1198-1204
- PubMed: 20944211
- DOI: https://doi.org/10.1107/S1744309109025196
- Primary Citation of Related Structures:
3H0N - PubMed Abstract:
The crystal structure of Jann_2411 from Jannaschia sp. strain CCS1, a member of the Pfam PF07336 family classified as a domain of unknown function (DUF1470), was solved to a resolution of 1.45 Å by multiple-wavelength anomalous dispersion (MAD). This protein is the first structural representative of the DUF1470 Pfam family. Structural analysis revealed a two-domain organization, with the N-terminal domain presenting a new fold called the ABATE domain that may bind an as yet unknown ligand. The C-terminal domain forms a treble-clef zinc finger that is likely to be involved in DNA binding. Analysis of the Jann_2411 protein and the broader ABATE-domain family suggests a role as stress-induced transcriptional regulators.
Organizational Affiliation:
Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, Menlo Park, CA, USA.