Dimer Formation of a Stabilized Gbeta1 Variant: A Structural and Energetic Analysis
Thoms, S., Max, K.E.A., Wunderlich, M., Jacso, T., Lilie, H., Reif, B., Heinemann, U., Schmid, F.X.(2009) J Mol Biol 391: 918-932
- PubMed: 19527728
- DOI: https://doi.org/10.1016/j.jmb.2009.06.031
- Primary Citation of Related Structures:
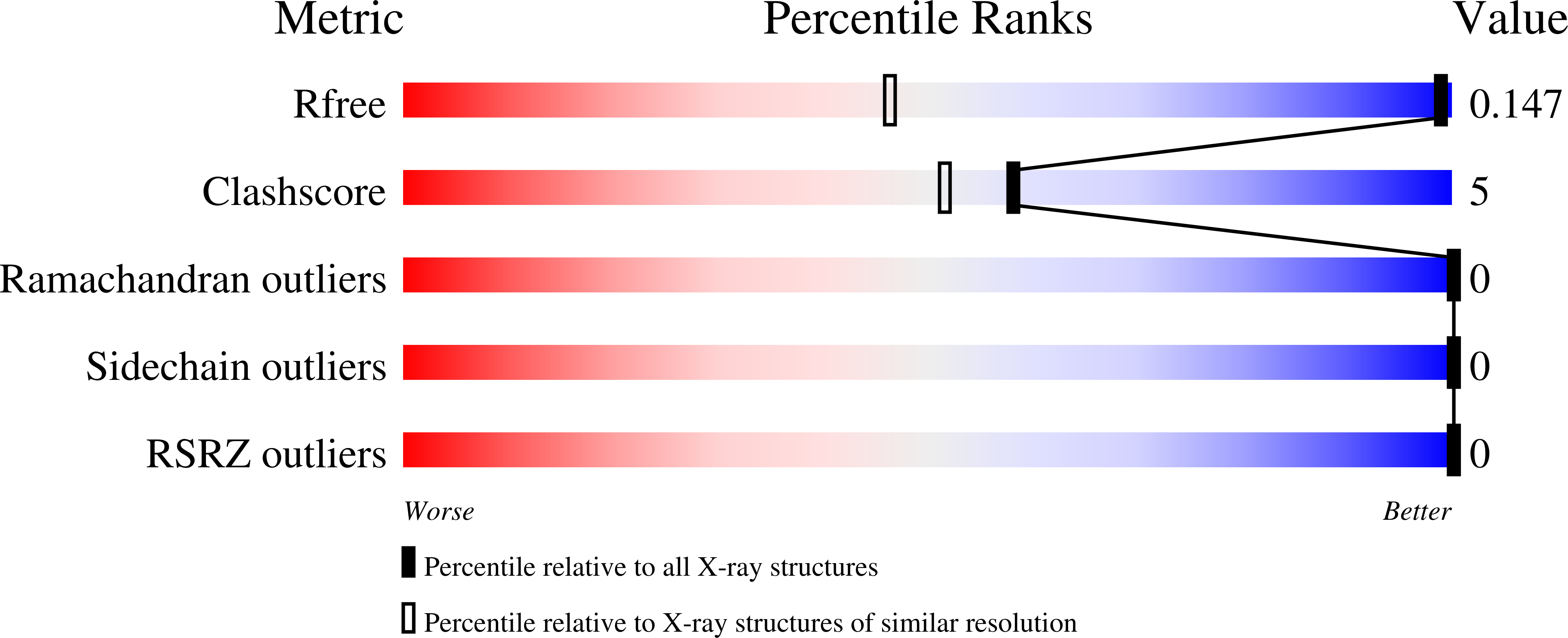
3FIL - PubMed Abstract:
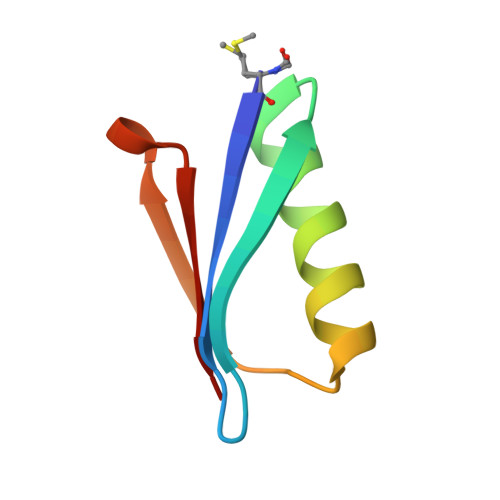
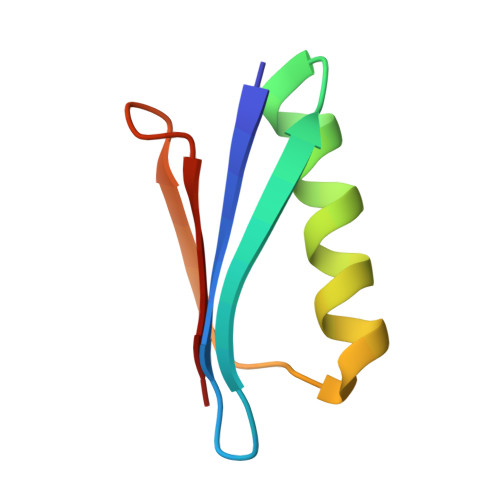
In previous work, a strongly stabilized variant of the beta1 domain of streptococcal protein G (Gbeta1) was obtained by an in vitro selection method. This variant, termed Gbeta1-M2, contains the four substitutions E15V, T16L, T18I, and N37L. Here we elucidated the molecular basis of the observed strong stabilizations. The contributions of these four residues were analyzed individually and in various combinations, additional selections with focused Gbeta1 gene libraries were performed, and the crystal structure of Gbeta1-M2 was determined. All single substitutions (E15V, T16L, T18I, and N37L) stabilize wild-type Gbeta1 by contributions of between 1.6 and 6.0 kJ mol(-1) (at 70 degrees C). Hydrophobic residues at positions 16 and 37 provide the major contribution to stabilization by enlarging the hydrophobic core of Gbeta1. They also increase the tendency to form dimers, as shown by dependence on the concentration of apparent molecular mass in analytical ultracentrifugation, by concentration-dependent stability, and by a strongly increased van't Hoff enthalpy of unfolding. The 0.88-A crystal structure of Gbeta1-M2 and NMR measurements in solution provide the explanation for the observed dimer formation. It involves a head-to-head arrangement of two Gbeta1-M2 molecules via six intermolecular hydrogen bonds between the two beta strands 2 and 2' and an adjacent self-complementary hydrophobic surface area, which is created by the T16L and N37L substitutions and a large 120 degrees rotation of the Tyr33 side chain. This removal of hydrophilic groups and the malleability of the created hydrophobic surface provide the basis for the dimer formation of stabilized Gbeta1 variants.
Organizational Affiliation:
Universität Bayreuth, Germany.