Crystal structure of the FGFR2 D2 domain
Brown, A., Blundell, T.L.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Fibroblast growth factor receptor 2 | 105 | Homo sapiens | Mutation(s): 0 Gene Names: FGFR2, BEK, KGFR, KSAM EC: 2.7.10.1 | 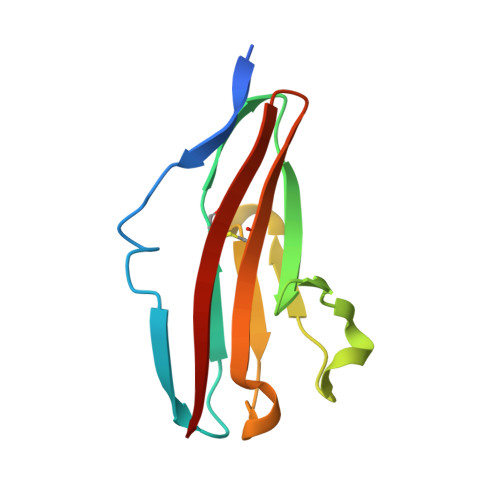 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P21802 (Homo sapiens) Explore P21802 Go to UniProtKB: P21802 | |||||
PHAROS: P21802 GTEx: ENSG00000066468 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P21802 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 41.87 | α = 90 |
| b = 78.24 | β = 90 |
| c = 85.9 | γ = 90 |
| Software Name | Purpose |
|---|---|
| MOSFLM | data reduction |
| SCALA | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| ADSC | data collection |