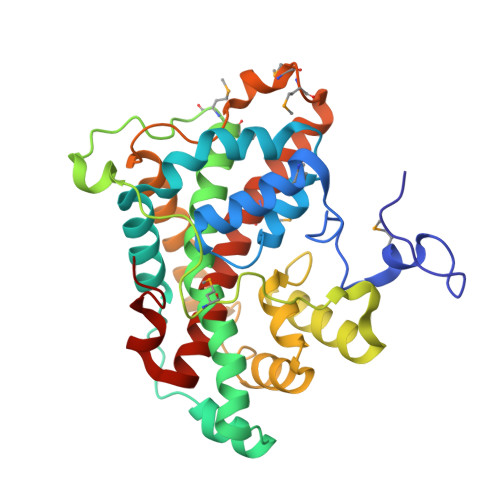
The crystal structure of DehI reveals a new alpha-haloacid dehalogenase fold and active-site mechanism
Schmidberger, J.W., Wilce, J.A., Weightman, A.J., Whisstock, J.C., Wilce, M.C.J.(2008) J Mol Biol 378: 284-294
- PubMed: 18353360
- DOI: https://doi.org/10.1016/j.jmb.2008.02.035
- Primary Citation of Related Structures:
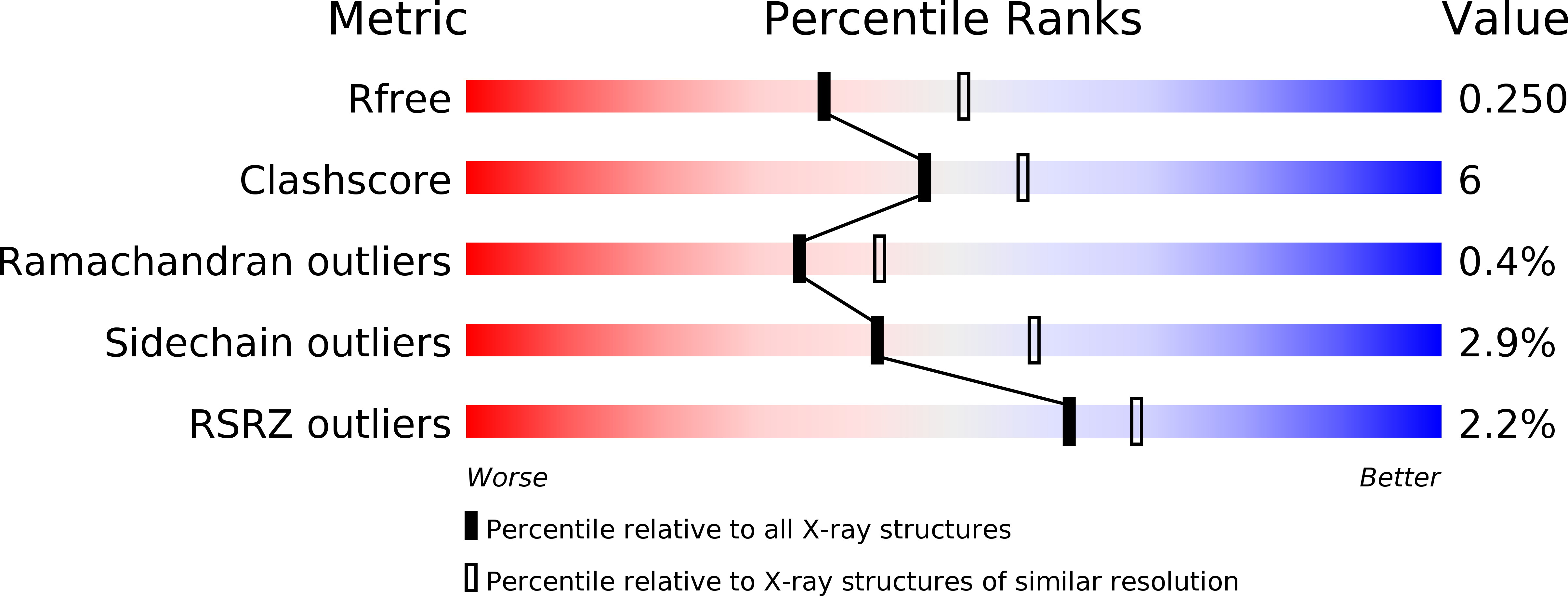
3BJX - PubMed Abstract:
Haloacid dehalogenases catalyse the removal of halides from organic haloacids and are of interest for bioremediation and for their potential use in the synthesis of industrial chemicals. We present the crystal structure of the homodimer DehI from Pseudomonas putida strain PP3, the first structure of a group I alpha-haloacid dehalogenase that can process both L- and D-substrates. The structure shows that the DehI monomer consists of two domains of approximately 130 amino acids that have approximately 16% sequence identity yet adopt virtually identical and unique folds that form a pseudo-dimer. Analysis of the active site reveals the likely binding mode of both L- and D-substrates with respect to key catalytic residues. Asp189 is predicted to activate a water molecule for nucleophilic attack of the substrate chiral centre resulting in an inversion of configuration of either l- or d-substrates in contrast to D-only enzymes. These details will assist with future bioengineering of dehalogenases.
Organizational Affiliation:
Department of Pharmacology, School of Medicine and Pharmacology, The University of Western Australia, Perth, Western Australia 6907, Australia.