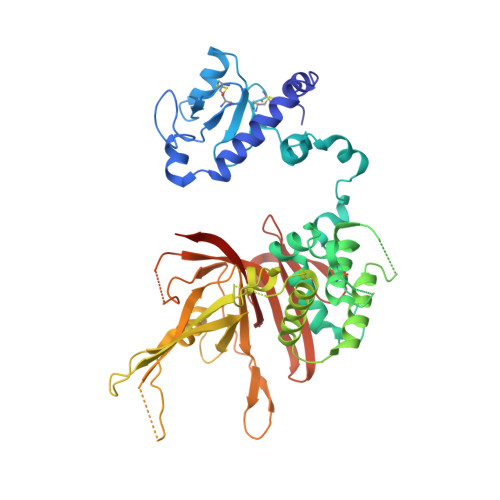
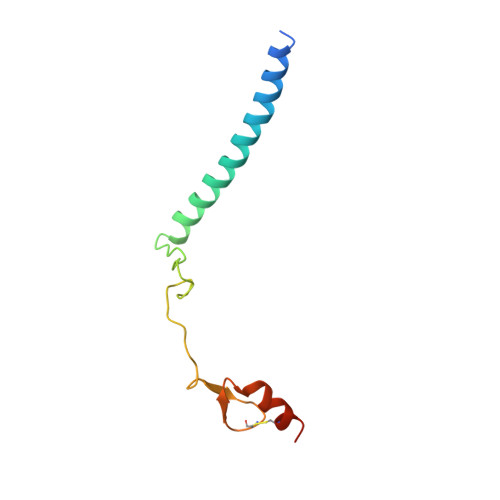
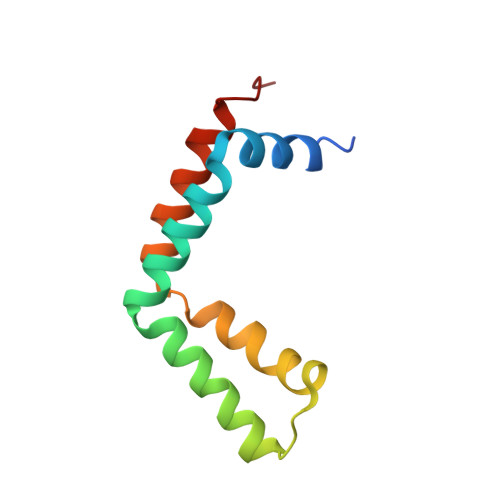
Structural basis for assembly and activation of the heterotetrameric SAGA histone H2B deubiquitinase module.
Kohler, A., Zimmerman, E., Schneider, M., Hurt, E., Zheng, N.(2010) Cell 141: 606-617
- PubMed: 20434206
- DOI: https://doi.org/10.1016/j.cell.2010.04.026
- Primary Citation of Related Structures:
3M99 - PubMed Abstract:
Deubiquitinating enzymes (DUBs) regulate diverse cellular functions by cleaving ubiquitin from specific protein substrates. How their activities are modulated in various cellular contexts remains poorly understood. The yeast deubiquitinase Ubp8 protein is recruited and activated by the SAGA complex and, together with Sgf11, Sus1, and Sgf73, forms a DUB module responsible for deubiquitinating histone H2B during gene expression. Here, we report the crystal structure of the complete SAGA DUB module, which features two functional lobes structurally coupled by Sgf73. In the "assembly lobe," a long Sgf11 N-terminal helix is clamped onto the Ubp8 ZnF-UBP domain by Sus1. In the "catalytic lobe," an Sgf11 C-terminal zinc-finger domain binds to the Ubp8 catalytic domain next to its active site. Our structural and functional analyses reveal a central role of Sgf11 and Sgf73 in activating Ubp8 for deubiquitinating histone H2B and demonstrate how a DUB can be allosterically regulated by its nonsubstrate partners.
Organizational Affiliation:
Biochemie-Zentrum der Universität Heidelberg, Im Neuenheimer Feld 328, 69120 Heidelberg, Germany. alwin.koehler@mfpl.ac.at